INTRODUCTION
The anterior cervical discectomy and fusion (ACDF) surgery has been widely carried out in the world as a standard treatment for degenerative cervical disc diseases2,9,23). The combination of various bone graft substitutes including autogenous bone, allobone, synthetic bone substitute as materials inserted into the interbody space after discectomy and the cage to maintain disc space has been performed in interbody fusion, and the stability of clinical and radiological outcomes has been proven through several theses11). Polyetheretherketone (PEEK) and titanium cages may be used as stand-alone for a single-level cervical disc disease, however the incidence of subsidence and non-union is high multi-level cervical disc diseases of 2 or more levels, making it difficult to expect stable bone fusion4,12,35,38). Therefore, rather than stand-alone cage fusion, surgery to transplant a bone substitute into interbody space by packing it in a spacer or cage and to induce stable bone fusion by reinforcing the fixation force with the plate and screw fixation in recent years10,33,40).
Allograft cervical spacer is the most commonly used implant material and shows a relatively stable fusion rate, however various problems such as spacer breakage, subsidence, dislodgement, and non-union occur in the process of bone fusion, resulting in relatively moderate clinical results such as delayed bone fusion and axial pain, as well as serious severe complications such as re-operation25,31,41).
NOVOMAX® spacer is a synthetic glass-ceramic spacer and has osteoactive material properties. It is considered an appropriate material for ACDF because it can form the stability of the operative segment at the beginning of fusion surgery by inducing an early tight bone bridge at the interface between the implant and the surrounding autogenous bone19). The purpose of this study is to compare the results of long-term follow-up of more than 2 years after surgery for ACDF patients using allograft spacer and NOVOMAX® spacer to identify the stability and efficacy of ACDF surgery using NOVOMAX® spacer.
MATERIALS AND METHODS
1. Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee (AJIRB-MED-MDB-19-263) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
2. Subjects
This is a prospective study that conducted a single-blind randomized controlled test on patients requiring surgical treatment due to degenerative cervical disc diseases from July 2014 to June 2015. All procedures performed are in this study in accordance with the ethical standards of the institutional research committee. All data were collected after obtaining patient consent. The inclusion criteria were the ages of 20 to 75, targeting patients who had a symptomatic degenerative cervical disc disease in the segment between C3-C7 (except C7-T1 level) and needed surgical treatment due to intractable radiculopathy or myelopathy and who showed no improvement in symptoms despite a 3-month conservative treatment and selected patients who could be followed up for at least 24 months after surgery to conduct a study. Trauma-related disc diseases, translation of ≥3.5 mm, sagittal angulation of ≥20 degrees, history of previous cervical spine surgery, long-term use of steroid which may reduce the bone quality of cervical spine and affect the results of clinical trials, infectious condition, metabolic diseases related bone quality and tumorous condition were excluded from the study (Table 1).
3. Operation Technique
Enrolled patients were divided into 2 groups. An allograft spacer (KBB, Seoul, Korea) was used for Group 1, and NOVOMAX® cervical spacer (CGBio, Seoul, Korea) was used for Group 2 to perform conventional ACDF. All patients were treated using a standard Smith and Robinson’s34) anterior cervical approach by 1 neurosurgeon2). After the completion of discectomy and any necessary decompression for a neural structure under a microscope, the cartilaginous endplates were removed. Endplates were cleaned and fashioned with curettes and a high-speed pneumatic drill, and the endplate was preserved as much as possible to prevent cage subsidence. After intervertebral space was widened sufficiently using Caspar screw, an appropriate-size allograft spacer packed with a demineralized bone matrix was inserted without impact on the spacer itself to avoid the breakage or crack in Group 1, and an appropriate-size NOVOMAX® cervical spacer was inserted in Group 2. Among patients, only one type of spacer was used for multilevel surgery. And each spacer used in surgery had the same width and depth, and only the height was different (6-7 mm). And then a rigid anterior cervical spine plate was applied in all cases. After removing the Caspar screw, the cervical plate with appropriate length was fixed by the method that fixing screws (length, 18 mm) to upper and lower levels were achieved upward and downward respectively to the oblique direction of the vertebral bodies. Before closing the superficial layers, a lateral radiograph was obtained, and the correct position of the implant and over-distraction was checked by comparison with the preoperative image. All patients were treated with the same protocol after surgery and consisting of immobilization with the Philadelphia cervical collar for 4 weeks.
4. Material Properties
The material of NOVOMAX® spacer is CaO-SiO2-P2O5-B2O3 bioactive glass-ceramics, which has superior mechanical strength than the human bone (Table 2). Compared to human bones, BGS-7 is more than twice as likely to be compressive strength and bending strength except fracture toughness. Therefore, it is expected that the NOVOMAX® spacer shows a lower incidence of the breakage after surgery than the allograft spacer. To confirm the biomechanical characteristics of the NOVOMAX® spacer and the allograft spacer, the finite element analysis of both spacer using the test conditions of the ASTM F2077 (test methods for intervertebral body fusion devices) was performed. The stresses were well distributed at both NOVOMAX® spacer and the allograft spacer, but the peak stress of the allograft spacer was relatively high comparing the strength of the human bone. Contrary, the peak stress of the NOVOMAX® spacer was relatively low (Fig. 1). Furthermore, the strength of the allograft spacer could be lower than the intact human bone because of the drying process for the sterilization of the products5). Therefore, it would seem that the NOVOMAX® spacer has superior biomechanical stability than the allograft spacer.
5. Outcome Measures
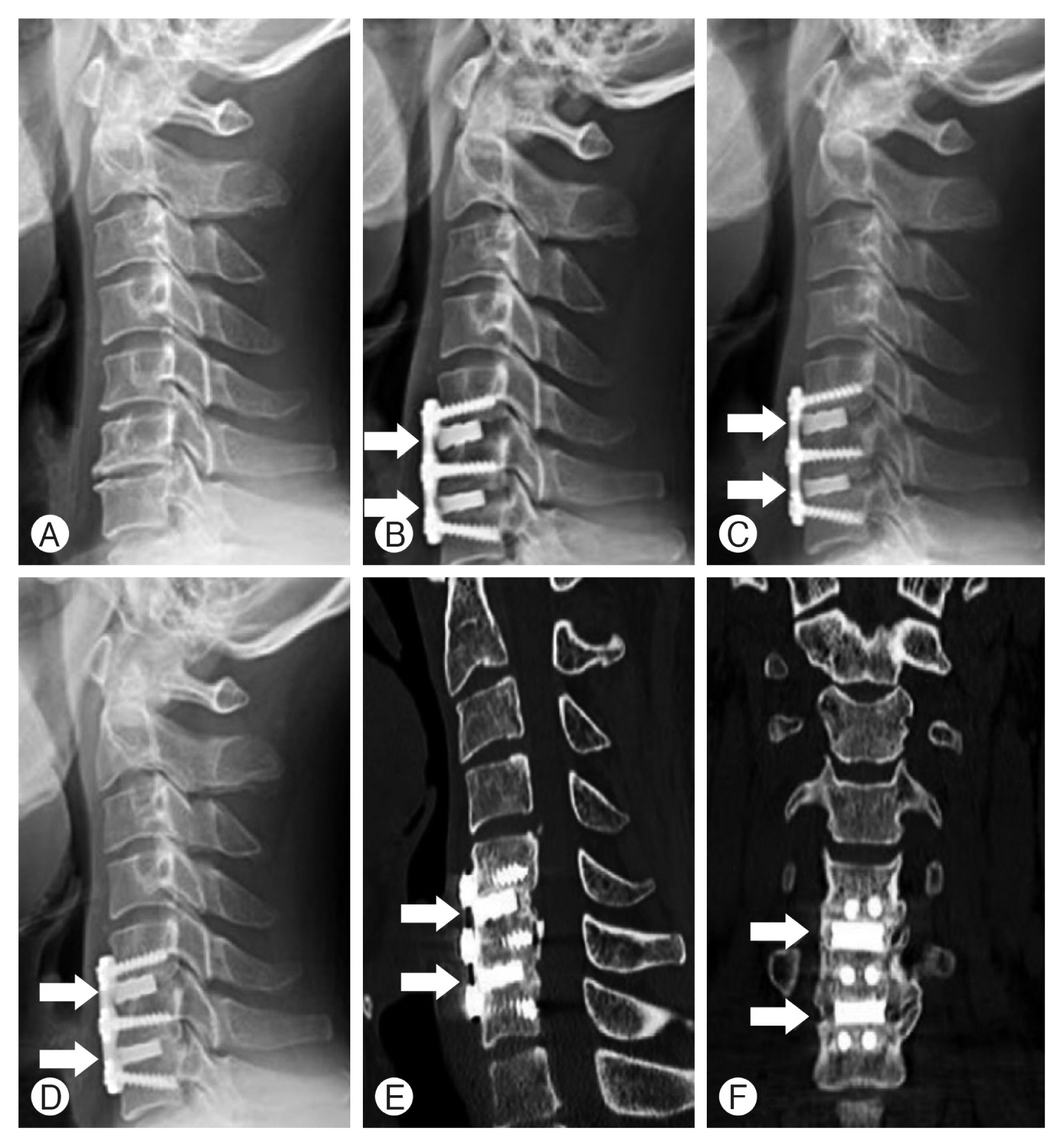
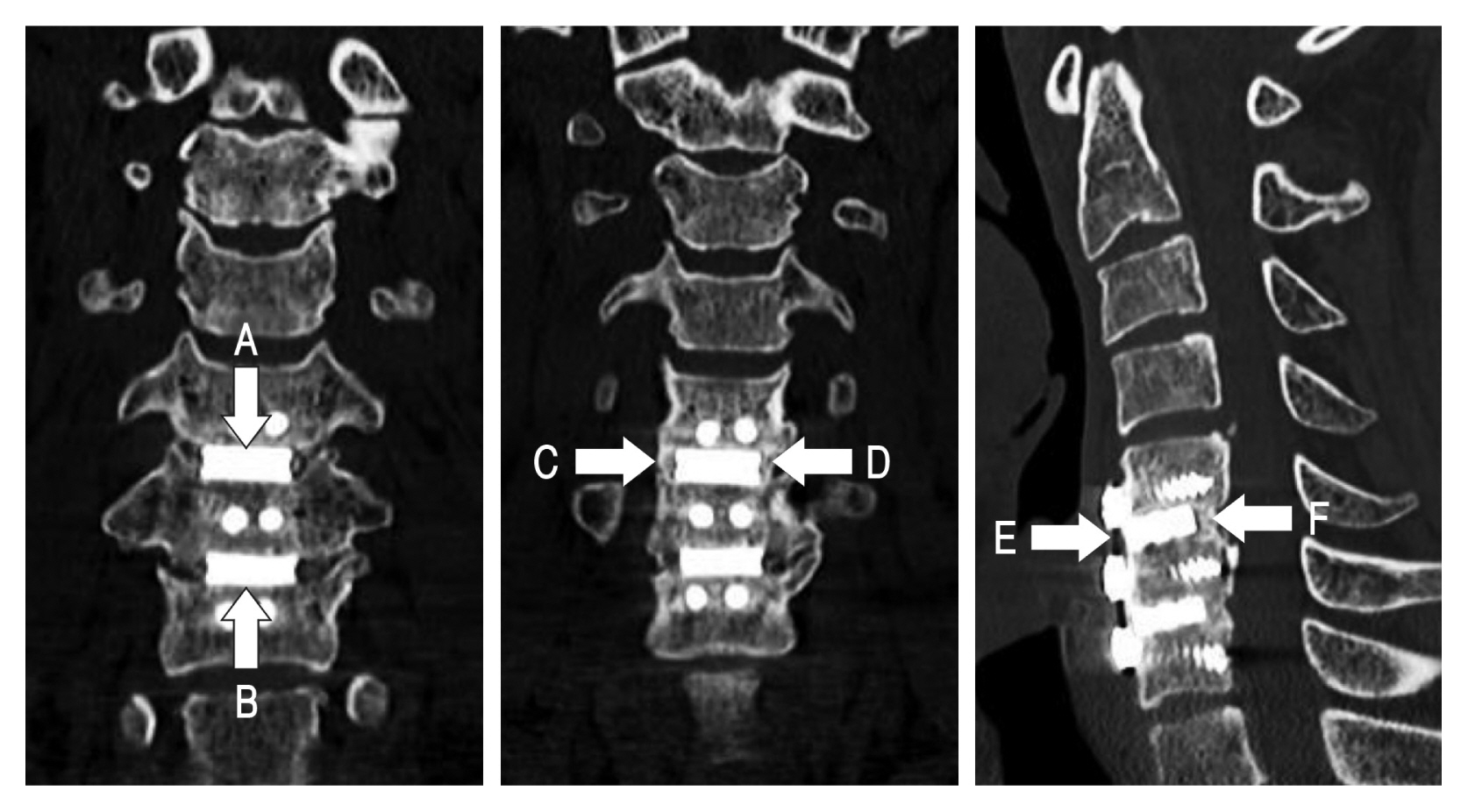
Radiologic evaluation was performed on all patients with preoperative plain X-ray images of cervical spine anteriorposterior (AP), lateral, flexion, and extension views, computed tomographic (CT) scan, and magnetic resonance image. After surgery, plain X-ray images of cervical spine AP, lateral, flexion, and extension views were taken 1, 3, 6-months, and every 1 year after surgery. At 12 and 24 months after surgery, cervical spine CT images were taken to confirm bone fusion clearly in comparison with before surgery (Fig. 2). Bone fusion was checked by 2 radiologists to reduce bias.
Plain X-ray images and CT were used to judge the bone fusion with a 6-point scoring system as an indicator to confirm the bone fusion proposed by this author in the previous study (Fig. 3)13). Subsidence is defined as the sinking a body with a higher elasticity modulus (e.g., graft, cage, spacer) in a body characterized by a lower elasticity modulus (e.g., vertebral body), resulting in 3-dimensions changes of the spinal geometry 17). Subsidence was defined as a decrease of ≥3 mm of anterior or posterior disc height from that measured on the immediate postoperative radiograph. A definition of spacer breakage was defined as definite recognition of linear fracture or fracture particles of allograft spacer or NOVOMAX® spacer on postoperative radiograph during follow-up period.
Clinical outcomes included patient-reported neck pain, measured using the Visual analog scale (VAS), ranging from 0 to 10. Disability was assessed with the neck disability index (NDI), which uses scores ranging from 0 to 100 to reflect the degree of disability in patients with neck pain, where higher scores represent more severe disability.
RESULTS
1. Patients Demography
Only patients who could be followed up for at least 24 months after surgery were enrolled in the study. Thirty-four patients and 17 patients were included in the Group 1 and Group 2, respectively. The male to female ratio was 18 vs. 16 in Group 1 and 12 vs. 5 in Group 2, and the mean age was 60.8 years old in Group 1 and 61 years old in Group 2 (Table 3). The follow-up periods were 32 months and 30 months for Group 1 and Group 2, respectively. In Group 1, 24 patients (71%) underwent surgery for 1 segment, 8 patients (24%) for 2 segments, and 2 patients (6%) for 3 segments, and ACDF using allograft spacer was performed in a total of 46 segments. In Group 2, 5 patients (29%) underwent surgery for 1 segment, 9 patients (53%) for 2 segments, and 3 patients (18%) for 3 segments, and ACDF using NOVOMAX® spacer was performed in a total of 32 segments. Allograft spacer was mainly used in surgery of 1 segment (71%), and NOVOMAX® spacer was mainly used in multi-segment surgery of 2 or 3 segments (71%; Table 3). There were no statistically significant differences in age, follow-up period, fusion levels, and bone mineral density between the 2 groups.
2. Radiologic Outcome
It has been confirmed that all the other patients in both groups, except one case in Group 1, were fuses within 24 months after surgery. In Group 1, allograft spacer related complications occurred in 34 patients (74%): fusion failure occurred in 1 case, screw pull-out occurred in 1 case, and spacer related complications occurred in a total of 32 segments (70%) with dislodgement of 2 segments, breakage of 6 segments, subsidence of 24 segments (Fig. 4). In Group 2, NOVOMAX® spacer related complications occurred in 6 patients (19%): screw pull-out occurred in 1 case, and spacer-related complications occurred in 5 segments (16%) with dislodgement of 1 segment and subsidence of 4 segments (Table 4).
3. Clinical Outcome
The preoperative mean NDI score was 76.9±3.41 in Group 1 and 76.2±0.97 in Group 2 (p=0.270). The mean NDI score was at 1-month follow-ups (23.4±5.03 and 20.70±1.57, p=0.036), 3-month follow-ups (13.58±8.14 and 7.53±2.87, p<0.001), 6-month follow-ups (11.70±7.82 and 6.94±1.75, p=0.002), 12-month follow-ups (9.06±8.27 and 5.88±1.32, p=0.035), 24-month follow-ups (6.59±5.01 and 5.88±1.31, p=0.446) (Fig. 5).
The preoperative mean VAS score was 8.17±0.62 in Group 1 and 8.76±0.44 in Group 2 (p=0.001). The mean VAS score was at 1-month follow-ups (1.82±0.90 and 1.70±0.77, p=0.648), 3-month follow-ups (1.85±1.01 and 1.47±0.51, p=0.081), 6-month follow-ups (1.44±1.10 and 1.17±0.39, p=0.219), 12-month follow-ups (1.29±0.97 and 1.17±0.39, p=0.542), 24-month follow-ups (1.15±0.82 and 1.18±0.39, p=0.89) (Fig. 6).
There was no statistically significant difference in the NDI and VAS scores in either group. The NDI and VAS scores showed a significant decrease immediately after surgery compared to before surgery in both groups (p<0.001) and remained stable until the time of the last outpatient follow-up.
DISCUSSION
As a surgical treatment for degenerative cervical disc disease, ACDF is the most frequently performed operation in the world as the gold standard treatment for a long time. This is because ACDF can provide stable and positive results such as decompressing the spinal cord and nerve roots, maintaining the stability of the cervical spine, and restoring cervical alignment and lordosis8,34). As for the ACDF method, the implantation of the cervical cage or spacer can restore and maintain the intervertebral disc height and neural foramina height. The cagealone fusion of the single segment has been reported not to be inferior in the clinical progress in comparison with ACDF combining both cage or spacer and plate and screw fixation, however in the case of multiple segments, ACDF combining both cage or spacer and plate and screw fixation shows more stable clinical and radiologic results than cagealone fusion1,6,14,39).
A plate can be used with cages or spacers made of various materials such as titanium, PEEK, and allobone and can be used by packing bone materials inside10,32,40). In some cases, it is used only as a spacer made in a mesh form and inserted simply. In the case of cage shape, it is made in the form of a rim having space for inserting the bone material, and the axial load is transmitted only to the rim. Therefore, breakage and subsidence are more likely to occur. Cage or spacer related complications are affected by affinity to a bone of cage or spacer material itself, fragility, compression caused by normal cervical movement, rotation, durability against shearing force, area of the cage or spacer in contact with the end-plate to which the axial load is transferred, fast bone bridge formation at the implant-to-bone interface at the recipient site. Since the cage-shaped implant only serves as a frame to maintain the intervertebral space, an additional bone substitute to fill the inside is required. Titanium cage has an affinity with the bone, however it requires a long period of time to obtain bone formation at the bone to metal interface and the resulting stability and show the pattern of fusion as part of the molding of the surrounding bony structure proceeds such as subsidence, bony erosion of contacted end-plate due to significantly higher strength than that of bone during the period19). PEEK is relatively close to the strength of the bone however, it has no affinity with bone. And it also takes a long time to form the bone to implant interface, so molding, subsidence of the surrounding bony structure occurs in almost all cases during the period from the beginning of surgery to bone formation19). Traditionally, allobones have been used for fusion, which provides both osteoconductive and osteoinductive environment that results in very high fusion rates, but has disadvantages. The cortical bone itself is already broken, even with a small impact of malleting during implant insertion leading to invisible linear fracture lines forming, causing implant dislodgement, breakage, and subsidence more frequently than other types of implants during the follow-up period (Fig. 4)25,31,41). Pirkle et al.29) analyzed 6,130 patients who underwent ACDF with allograft (4,063 patients) or intervertebral cages (2,067 patients) to compare non-union rates after 1 year postoperative. Cages were unable to be classified by type and may have been “PEEK, titanium, mesh, or porous”. Overall non-union rates were significantly higher in the cage group (5.32%) than in allograft group (1.97%; p<0.0001). Therefore, we used bioactive ceramicglass cages to complement the disadvantage of other cages, and to confirm the clinical and radiologic outcome of these cages.
There is a report that clinical and radiological outcomes are stable only with empty cage insertion, but this corresponds to a single segment, and there are problems as a generally accepted surgical method because the formation of a stable bone fusion requires a long period of more than one year, it is difficult to obtain early spinal stability and it is also difficult to expect stable bone fusion formation32,36). The plate and screw fixation forms stable bone fusion during long-term follow-up in many cases, and clinical radiologic results show relatively stable results compared to other implants. However, bone fusion is formed when the part of the intervertebral space is collapsed, resulting in loss of segmental lordotic curve and loss of lordotic curve of the entire cervical spine3,7,8,37). In our study, the allograft spacer group improved fixation and stability with a plate and screw system after inserting the spacer, non-etheless, the unfavorable radiologic outcome was seen in this group compared to the NOVOMAX® group. In Group 1, allograft spacer related complications occurred in 34 cases (74%), whereas in group 2 in 6 cases (19%). In particular, subsidence occurred in 52%, while only around 13% in Group 2, showing better results. However, many authors support statements that implant subsidence does not significantly affect the patient’s pain or the quality of daily life24). Perhaps, this result comes from preserving the disc space after surgery to reduce irritation to the nerve.
In our study, ACDF surgery using NOVOMAX® spacer is multilevel but has fewer side effects (19%) and superior clinical improvement. NOVOMAX® spacers bind chemically to the bone forming an apatite layer15,27), which results in improved bone-bonding strength18). Among the many types of bioactive glass ceramics, CaO-SiO2-P2O5-B2O3 glass ceramics demonstrate high bioactivity and osteoconductivity17,21) and induce osteoblastic differentiation of human mesenchymal stem cells22). NOVOMAX® spacers show high osteoconductivity and mechanical strength and faster resorption rates20). NOVOMAX® spacers shows 2-fold higher mechanical strength than hydroxyapatite16). Furthermore, previous in vitro and in vivo studies show that NOVOMAX® glass-ceramics possesses high bioactivity and chemical bonding ability16-18,21,22). NOVOMAX® spacer is a true spacer that fills the entire intervertebral space without empty space with the largest area without insertion of other bone materials, and stable bone bridge (membrane) formation and resulting segmental stability can be expected in the early stage because the entire cross-sectional area is in contact with the end-plate, very stable force distribution can be achieved for the mechanical forces transmitted to the index level, which is the surgical segment according to the movement of the cervical vertebrae, lordotic angle and height of intervertebral space are maintained due to pre-designed segmental angle, high strength not broken in malleting and the material itself has bone-friendly properties. More stable clinical and radiological outcomes can be expected in the early stage of recovery after surgery compared to the cages inserted into the intervertebral disc space that has been used in the past. It is also considered to be an ideal material to complement the disadvantages of hydroxyapatite, which is excellent in bone conduction ability but poor in biodegradability and tricalcium phosphate, which is biodegradable but has poor bone-forming ability30).
Our study has some limitation. First, there were only 51 patients included in the study, which is a relatively small number of patients. Thus, further large-scale, prospective, comparative studies are required. Unfortunately, patients who were not able to be followed up after surgery were excluded. Second, our study has subject to selection bias due to material properties and the absence of randomization. The allograft spacer tended to be mainly used in the one-segment surgery and NOVOMAX® spacer in the multi-segment surgery. Furthermore, studies are needed, including more patients, depending on the number of levels in each spacer.
CONCLUSION
ACDF using NOVOMAX® spacer well maintains the height of the intervertebral space of the operative segment as well as the decompression of neural structure and is recommended as surgical treatment of safe and efficient degenerative cervical disc disease which can expect stable radiological results through the early stable bone formation. However, there is no statistical difference between radicular pain, axial pain in the 2 groups.