INTRODUCTION
Yaşargil12) has brought a paradigm shift to neurosurgery with the use of the microscope. Recent advances in radiosurgery and endoscopic surgery have challenged in microscopic surgery in the neurosurgical field. However, the more these challenges, the more experienced and technologies are being accumulated and developed. Through this, the accuracy of surgery is gradually improving. In addition, navigation systems have improved accuracy during surgery and indocyanine green (ICG) angiography has rendered the process easier4,7,11). Furthermore, intraoperative computed tomography (CT) and magnetic resonance imaging (MRI) have been used to provide safer and more accurate treatment for patients5,8,10). Herein, our experience of surgery on a patient using the above-mentioned modalities with real-time ultrasound is reported.
CASE REPORT
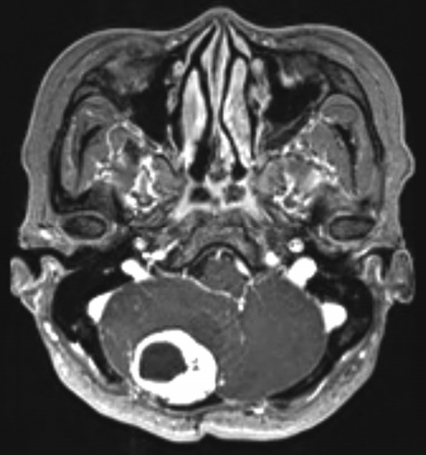
The patient underwent surgery in 2013 for a posterior fossa hemangioblastoma. There was no recurrence after gross total resection until a 2-year follow-up MRI. And the patient lost to follow-up. The patient revisited the outpatient department 2 years later due to a recent experience of dizziness and ataxia. The MRI showed local recurrence of hemangioblastoma (Fig. 1); therefore, a reoperation was planned.
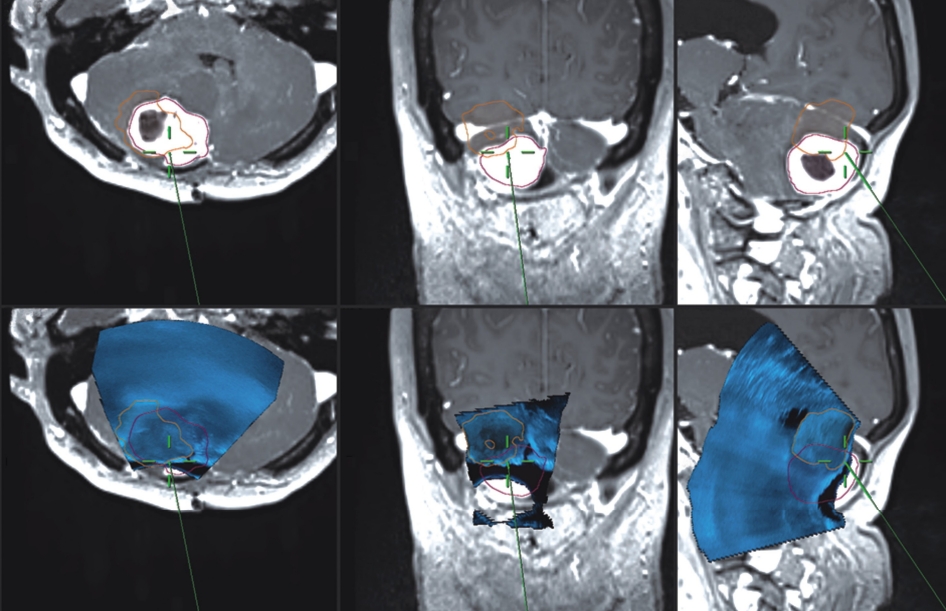
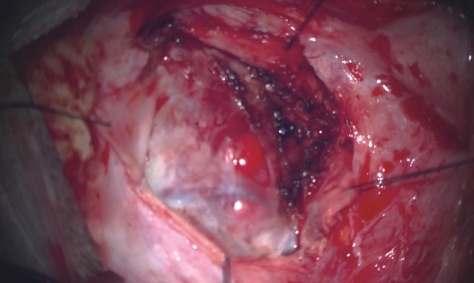
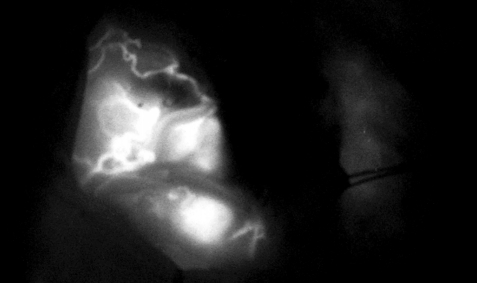
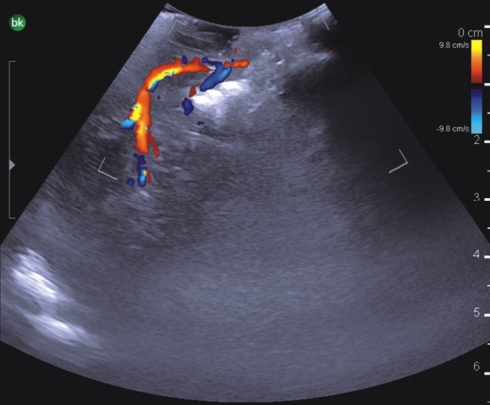
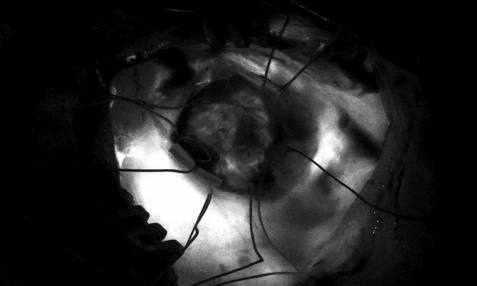
Sub-occipital craniectomy was performed with the patient in the concord position. Upon opening the dura adhesion with arachnoid membrane, cerebrospinal fluid (CSF) was gushed out and the cerebellum was sunken down causing displacement of the cerebellum and tumor; the location was different from that identified using the navigation system. The location of the tumor was confirmed using real-time ultrasound. Three-dimensional (3D) reconstruction was performed with the ultrasound to overlap the navigation system and confirm the location of the tumor. After the CSF was drained, it was confirmed that the tumor was displaced by as little as 1 cm and as much as 3 cm compared to the preoperative MRI (Fig. 2). The extent of the tumor was confirmed, and the dura was widely opened to expose the tumor. Under the microscope, many red vessels were observed on the surface of the tumor (Fig. 3). The vascularity of the tumor was identified using ICG angiography. The tumor was hypervascularity, however, determining the exact position of the feeding artery was difficult due to obstruction by the cerebellar parenchyma (Fig. 4). We used real-time doppler ultrasound to find the feeding artery to the superior margin of the tumor, and there were no other feeders (Fig. 5, Supplementary Video 1). After blocking the main feeding artery passing over the tumor superiorly, ICG angiography was performed again. The vascularity was significantly reduced (Fig. 6). It was judged that all the main feeders were blocked and coagulated, and the tumor was removed en-bloc. After controlling bleeding at the tumor removal site, the operation was completed with primary wound closure. There were no adverse events during the surgery. The gross total resection of the tumor was confirmed on postoperative MRI (Fig. 7). The patient was discharged without any other complications.
DISCUSSION
Neurosurgery is a complicated field of surgery and requires elaborate work with the major focus being on patient safety. Various modalities such as intraoperative neuro-monitoring, neuronavigation system, fluorescence, and ICG angiography are used. Intraoperative CT and MRI aid in reducing various risks that may occur during surgery5,8-10). However, examination of CT and MRI scans require a considerable amount of time and scans cannot be performed during surgery. In addition, cost-effectiveness is debatable3). Real-time ultrasound can help overcome these problems. It can be applied at a relatively low price, and results can be obtained by conducting inspections in a short time. Recently, several studies have introduced surgery using ultrasound. Manfield and Yu6) reported that the ultrasound-guided external ventricular drainage is very feasible, and Della Pepa et al.1) reported that the meningioma feature could be predicted using ultrasound. Dellaretti and Ronconi2) Introduced arteriovenous malformation surgery using doppler ultrasound. As in the present case, real-time ultrasound can help identify vascular lesions allowing early identification of the arterial feeders, thus providing the surgeon with an overall impression of the flow dynamics. In posterior fossa surgery, as in this particular case, the brain shift due to the CSF drain is inevitable. The ultrasound probe is larger in size than the conventional microsurgical instrument, so there is a limit to work in the operative field frequently. However, the use of neuronavigation solves the problem of the bulky ultrasound probe. Once a 3D image is constructed with an ultrasound probe and overlayed on the MRI of the navigation system, the overlayed ultrasound image can be checked simply using the navigation probe. It can perform image-guided surgery in real-time not inferior to intraoperative MRI. The combination of neuronavigation and Real-time ultrasound for resection of posterior fossa tumor resection can provide valuable intraoperative information of the location and resection level of the lesion, so it can maximize the extent of resection of the tumor and minimize the complication.