INTRODUCTION
When a central nervous system (CNS) lesion is found in patients with chronic lymphocytic leukemia (CLL), it is rarely diagnosed as CNS involvement of leukemic cells, which is known to occur mostly in cases of acute lymphoblastic leukemia. Richter’s syndrome (RS) refers to the transformation of CLL to diffuse large B-cell lymphoma (DLBCL), which occurs in 90% to 95% of cases or other aggressive lymphomas15). Otherwise, other CNS malignancies, non-tumorous diseases, and so on can be considered. RS is rare and it occurs in approximately 5% of CLL cases10). The CNS involvement of RS can also be considered if the CNS lesion is confirmed to be a DLBCL. CNS RS commonly occurs with latency in the preexisting CLL or with systematic RS13). We introduce an extremely rare case of an isolated CNS DLBCL without other systemic RS at the time of the initial diagnosis of CLL, which was at first confused as a CNS involvement of CLL.
CASE REPORT
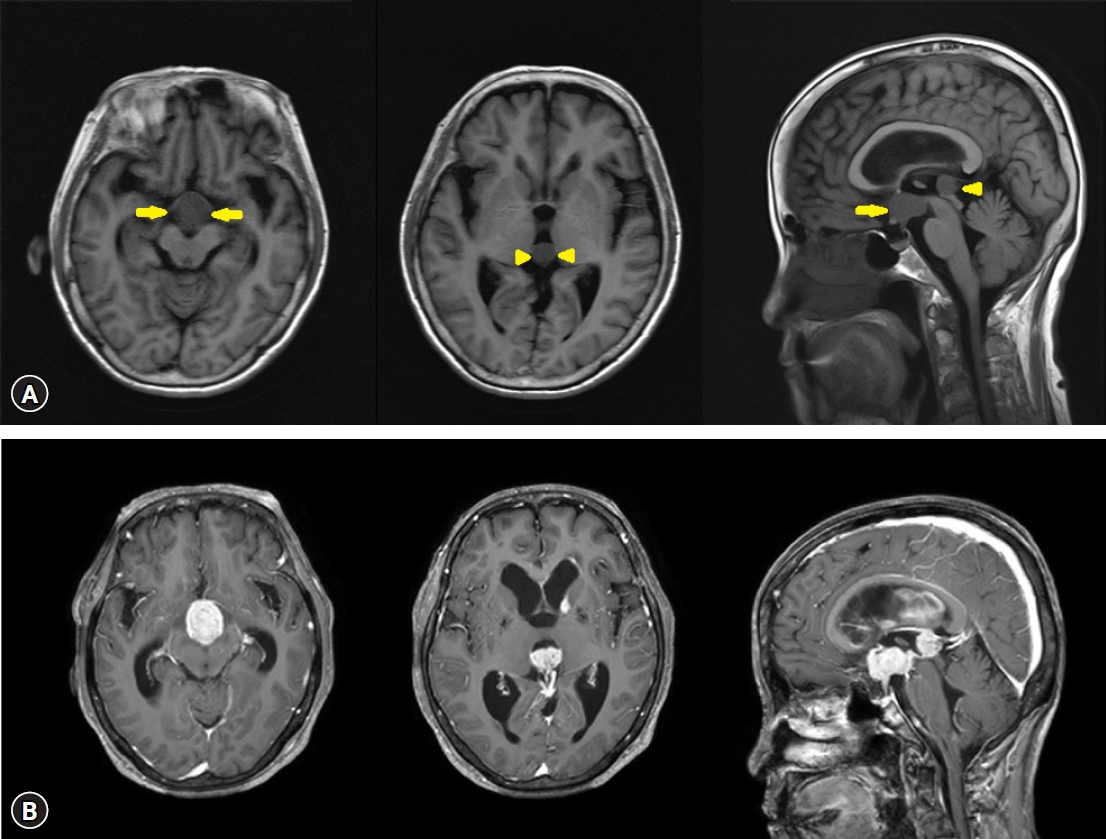
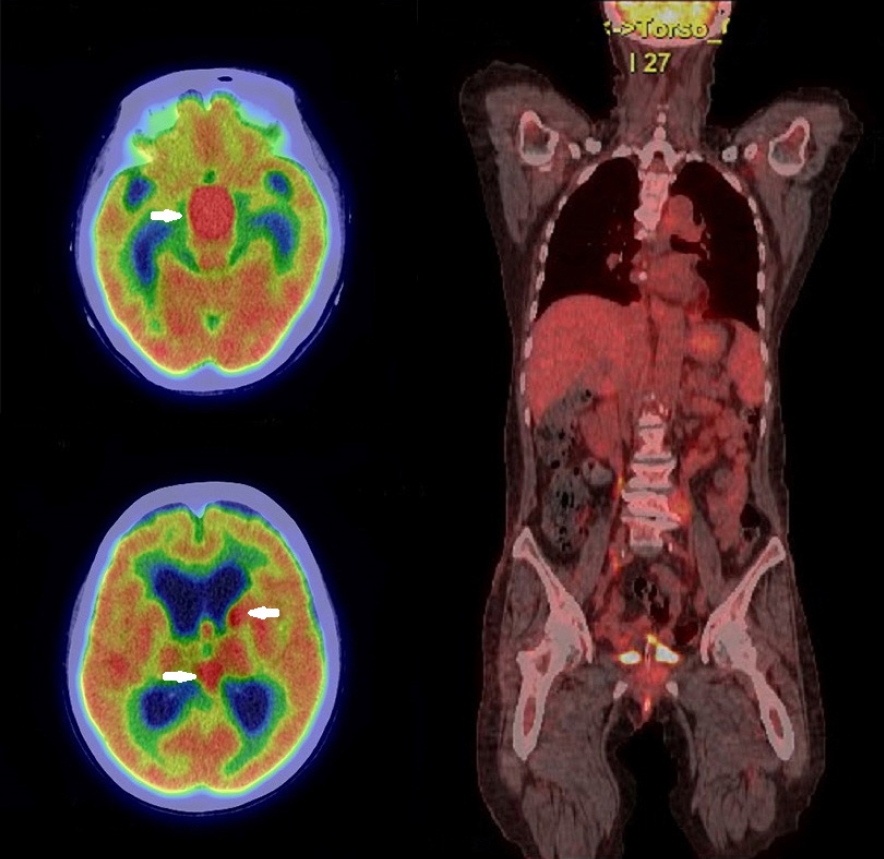
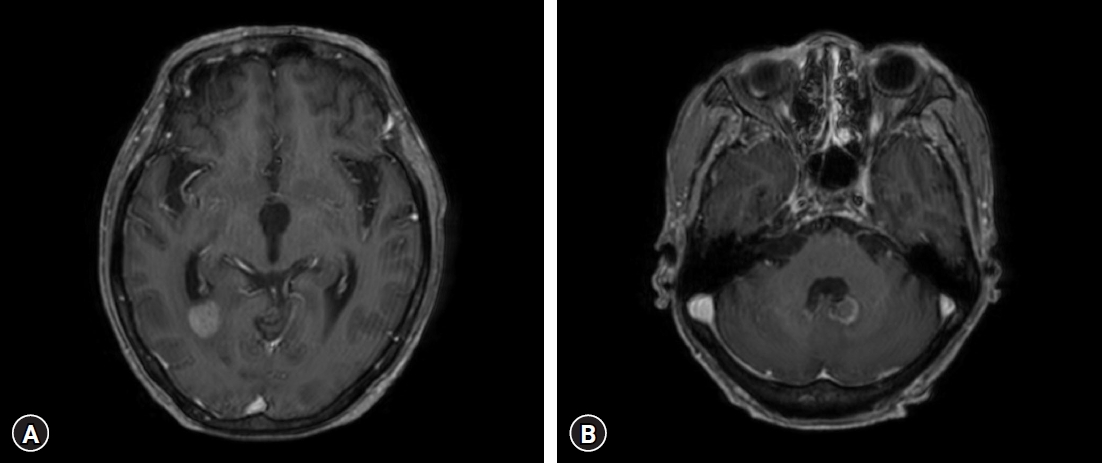
A 73-year-old woman with a history of diabetes mellitus and hypertension visited our hospital because of elevated white blood cell count and intraventricular masses (Fig. 1A), which were detected during a medical examination conducted at another hospital with a headache. Also, she has been complaining of general weakness but without a specific neurological deficit. In our examination, her white blood cell count was 129,800/mm3 with 90% lymphocytes, hemoglobin of 12.7 g/dL, hematocrit of 37.8%, platelet count of 157 × 109 /L, and lactate dehydrogenase of 226 IU/L (normal range, 120-250 IU/L). Her serum sodium level was very low at 119 mEg/L, and the basal hormonal study revealed panhypopituitarism. Her body temperature was 36.4°C. Brain magnetic resonance imaging (MRI) with gadolinium enhancement showed a suprasellar mass with a size of 2.4 × 2.1 cm, a pineal mass with a size of 1.7 × 1.5 cm within the 3rd ventricle, and enhancements in the left caudate nucleus, septum pellucidum, left lateral ventricle wall, pituitary stalk, clivus, inferior wall of the 4th ventricle, anterior surface of the ponto-medulla (Fig. 1B). Compared to the non-enhanced brain MRI performed at another hospital 8 days prior, the recent MRI showed that tumors in the 3rd ventricle increased in size and progression of hydrocephalus. The systemic evaluation was performed including whole-body fluorodeoxyglucose (FDG) positron emission tomography (PET)/computed tomography (CT) and no hot uptake lesion was observed except the brain lesions (SUVmax 20.2) (Fig. 2). Lymph node (LN) enlargement or other extranodal lesions were not found.
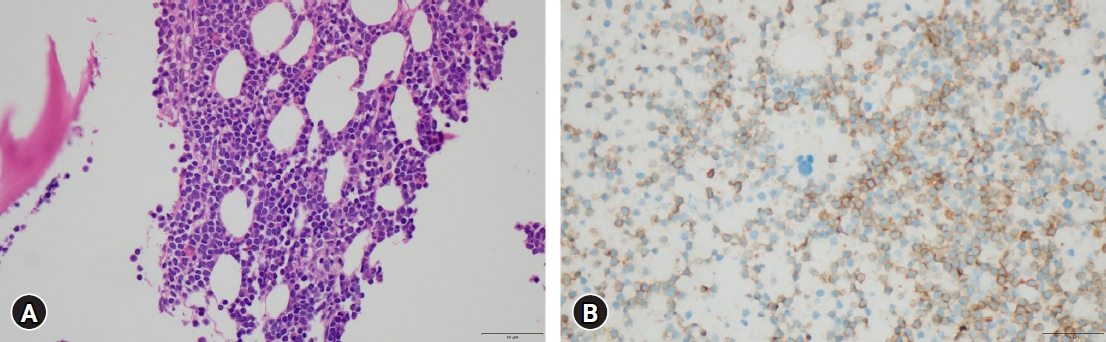
Peripheral blood smear presented severe leukocytosis made up of 90% of mature lymphocytes. Bone marrow (BM) biopsy revealed small mature lymphocytes with 80.6% being all marrow cells and concluded the development of CD5-positive small B-cell lineage CLL (Fig. 3A). Immunofluorescence exhibited CD3+(focal), CD5+, CD19+, CD20+, CD22+, CD45+, CD10-, CD23-, with ki-67 of 10% (Fig. 3B). A chromosomal study of BM lymphocytes showed an add 4p MAR, but without other CLL-related poor prognostic factors, such as deletion 17p, deletion 11q, or transformation.
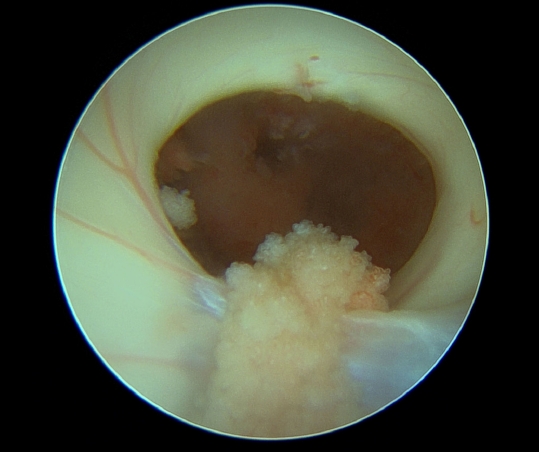
For the CNS lesions, CNS involvement of CLL, germ cell tumor, CNS lymphoma, and so on were considered differential diagnoses. Cerebrospinal fluid (CSF) analysis via lumbar puncture showed the following results: red blood cell count of 100/µL, white blood cell count of 300/µL (poly, 1%; mono, 99%), protein level of 143.87 mg/dL, and glucose level of 12 mg/dL, and α-fetoprotein, human chorionic gonadotropin, and Epstein-Barr virus (EBV) DNA-polymerase chain reaction showed negative findings. CSF cytology showed small atypical lymphocytes, which is most likely indicative of leptomeningeal involvement in CLL. Since the patient did not have LN adenopathy, anemia, thrombocytopenia, and hepatosplenomegaly, CLL corresponds to Rai stage 0 and Binet stage A (Table 1)1,18), both showing a low risk. With only aggressive involvement in CNS, it was necessary to ensure the CNS etiology. The patient underwent endoscopic biopsy for a suprasellar mass in the 3rd ventricle through the right Kocher's point and Ommaya reservoir implantation. On intraoperative finding, the mass in the 3rd ventricle appeared very hypervascular with a red surface (Fig. 4). Pathology result showed DLBCL (Fig. 5A). In situ hybridization for EBV was negative. Disconcordantly with BM, immunohistochemical (IHC) staining of the 3rd ventricle mass showed CD10+, CD20+, CD3-, CD5-, CD23-, CD30-, SOX11- Bcl2-, and Bcl6- with ki-67 of 80% (Fig. 5B).
With administering 4 mg of dexamethasone every 6 hr for 9 days, suprasellar and pineal masses had already begun to decrease in size at postoperative serial brain CT/diffusion MRI, even before the chemotherapy was started. The patient underwent 3 cycles of systemic high-dose methotrexate. After 3 months of treatment, generalized tonic-clonic seizure occurred and a follow-up brain MRI showed that all the preexisting lesions have disappeared, but new hemorrhagic masses of approximately 1 cm were found in the posterior horn of the right lateral ventricle (Fig. 6A) and in the left superior wall of the 4th ventricle (Fig. 6B). In the CSF analysis, atypical lymphoid cells were detected at every examination during the treatment period. Due to the patient's poor general condition, additional chemotherapy was not performed and palliative intensity-modulated radiation therapy (33 Gy/15 fx) for the whole ventricle was performed. The patient has been in a bed-ridden state due to general weakness since the initial diagnosis of CLL and suffered from medical comorbidities. She died 6 months after the initial diagnosis.
DISCUSSION
The incidence of CNS involvement in CLL is 0.4% to 2% of cases diagnosed with clinical symptoms and 7% to 71% of cases found in autopsy as asymptomatic, which is highly subclinically present20,23). The incidence of overall RS in CLL is known to be approximately 5%10). LN or BM infiltration at the time of large cell transformation is typical with/without extranodal involvement. Particularly, it is extremely rare that the site localized to a single extranodal site is CNS24,25). According to previous data involving 4,147 CLL patients from the Mayo Clinic, there were 15 (0.3%) and 18 (0.4%) patients diagnosed with isolated CNS RS and CNS involvement in CLL, respectively20). Of the 204 CLL RS patients, 4 patients (2.0%) had isolated CNS RS out of 11 (5.4%) patients with biopsy-proven CNS RS5). CNS involvement in both CLL and RS commonly occurs with latency in preexisting CLL13). It is extremely rare that isolated CNS RS is found simultaneously at the initial diagnosis of CLL, as our patient. Among the 19 isolated CNS RS cases reported by 2022, four were diagnosed simultaneously with the initial diagnosis of CLL7,21,24,25).
CNS involvement in CLL/RS can present with visual changes(22%), encephalopathy (29%), and weakness with paresthesia (22%)16). Symptoms are based on the location of the lesion, both favor spreading via leptomeninges. If CNS symptoms occur in CLL patients, CSF analysis, and imaging studies such as brain MRI, are performed for differential diagnosis. Whole-body FDG-PET can help evaluate the existence of any systemic involvement. In our patient, CSF cytology and brain biopsy results showed a discrepancy. Patients diagnosed only by CSF analysis and/or brain MRI without biopsy confirmation may have been misdiagnosed. The true incidence of CNS CLL/RS may be different from the already reported incidence. Among the 31 symptomatic CLL patients with CSF analysis demonstrating the presence of CLL B-cells, 18 (58%) had an alternative diagnosis after tissue biopsy. This included CNS infections in 8 patients, autoimmune/inflammatory process in 5, metastatic cancer in 1, and non-CLL-related etiology in 420).
The IHC staining showed that the CLL cells in BM and DLBCL on brain biopsy showed slightly different surface markers, indicating different immunophenotypes. If different immunophenotypes are shown, there are both possibilities originating from the identical B-cell clonality, or activating from different clonality, and the definition of RS broadly includes both of these cases22,24,25). The type of clonal pattern has an impact on prognosis; clonally related RS has a dismal outcome, is resistant to chemotherapy, and has a median survival of approximately 12 months, whereas clonally unrelated RS has a median survival of 65 months9,11). Unfortunately, in our patient, immunoglobulin heavy chain rearrangement studies were not performed on the BM and brain specimen; thus, we cannot determine whether BM CLL and the CNS DLBCL had identical clonality.
In the case of CNS involvement in CLL/RS, most cases occur over a period of latency in preexisting CLL13). However, our patient presented isolated CNS RS simultaneously when CLL was initially diagnosed. Compared to the relatively stable systemic CLL, only CNS lesions progressed rapidly and extensively. Of the 19 reported isolated CNS RS cases, data were available for 10 patients, including 8 with Binet stage A, 2 with Binet stage B, and no patient with advanced Binet stage7,21,25). Our patient had Binet stage A, Rai stage 0 without LN adenopathy, splenomegaly, and thrombocytopenia, but she did not have other suspected clinical RS symptoms, such as fever and elevation of serum LDH level. It may be difficult to suspect the first chance that isolated CNS RS occurred alone without other nodal/extranodal lesion13). Most studies suggest that there is no clear association between the Rai and Binet stages of CLL and CNS involvement in CLL/RS6,14,23). Although there is only a small number of cases, isolated CNS involvement, including our patient, seems to be independent of the CLL stage. However, if not limited to CNS, the advanced Rai/Binet stage is included as a risk factor for overall RS occurrence from CLL. Several studies have identified biological characteristics of CLL associated with an increased risk of RS: CD38, CD49d, and Zap-70 expression, unmutated IGHV, del11q, del17p, trisomy 12, complex karyotype, loss of cell cycle inhibitors CDKN1A/CDKN2A/CDKN1B, deletion of the p53/Rb/p27genes, mutations of the SAMHD1/XPO1/MED12/NOTCH1/MYC genes, overexpression of ZAP70/BCL2/LRP4 genes, decreased expression of MYBL1, and so on5,9,25).
Negative Bcl-2 and positive Bcl-6 are related to better outcomes in cases with CNS lymphoma2). Furthermore, the existence of MYD88L265P and CD79b mutation, which are the major genetic abnormalities of primary CNS lymphoma, provided a therapeutic target and better outcomes8,21).
It is known that long-term survival with a median survival of 111 months is possible among patients with normal karyotype CLL4). However, in the case of CNS involvement in CLL, a shortening of survival from 3 months to 3 years is observed6). A study showed a median survival of 12 (15 patients) and 11 (18 patients) months in the cohort with CLL and RS CNS involvement, respectively20). There was a trend toward better survival in those with CNS CLL as compared to those with CNS DLBCL13). In those with untransformed CLL presenting in the CNS, 14 of 23 (58%) patients were alive at the time of follow-up (median, 12 months), as compared to only 6 of 15 (33%) with RS and prior CLL alive at the time of follow-up (median, 3 months)13).
Aside from CNS involvement, RS itself is a fatal complication of CLL, with an average survival of 8 to 12 months3,12). A previous study of 204 patients with RS from CLL as confirmed by biopsy showed overall survival (OS) of 9.4 months for 15 CNS RS patients5). The median OS of 16 isolated CNS RS cases was 6 months, which was shorter than that for other sites of RS (8 months)19). Since most cases are diagnosed with CNS RS after a latency period with preexisting CLL, it is difficult to derive an average survival rate using only the data obtained from an extremely small number of cases, which had a simultaneous diagnosis of isolated CNS RS when CLL was initially diagnosed.
Our patient survived for 6 months after the initial diagnosis of CNS RS, which is thought to correspond to the conventional survival of CNS RS. Five CNS RS patients who concurrently had CLL also showed a median OS of 5 months13). In addition to the survival rate itself, it is thought that neurological deficits due to CNS lesion reduced the patient's performance status, limiting the application of other treatments and affecting the patient's quality of life throughout his/her lifetime.
Both CLL and RS CNS involvement are rare entities, and no gold standard treatment or first-line regimen has been defined. The treatments include intrathecal/intravenous chemotherapy, radiation therapy, and stem cell transplantation. Established therapy for primary CNS lymphoma can be applied to treat CNS RS, which includes intravenous/intrathecal high-dose methotrexate combined with rituximab (chimeric anti-CD20 monoclonal antibody). Additionally, an intrathecal regimen, such as methotrexate, cytarabine, and corticosteroid, can be used, which is the treatment for leptomeningeal diseases in CLL7,16). Chemoimmunotherapy with the combination of fludarabine, cyclophosphamide, rituximab, ibrutinib (a selective inhibitor of Bruton tyrosine kinase), and/or Venetoclax (a selective inhibitor of BCl-2) may be considered17). One patient underwent 1 cycle of temozolomide and whole-brain radiation therapy without conventional chemotherapy due to old age. She survived for approximately 11 months from the initial diagnosis of CLL with concurrent CNS RS7). Treatment effects can be determined by image and CSF clearance. The results are generally disappointing because of the chemoresistance and poor performance status of patients to continue the therapy25). CNS RS, especially isolated CNS RS, is a rare entity and more case collection and analysis will help establish its future treatment strategy.
CONCLUSION
In CLL patients, both CLL and RS (DLBCL) CNS involvement are rare, and the presence of CNS RS with the initial diagnosis of CLL is extremely rare. Both diseases can be difficult to distinguish in terms of B-cell lymphocytes. Appropriate treatment should be selected through rapid and exact CNS tissue diagnosis.