Adult Intramedullary Mature Teratoma of the Spinal Cord: An Unusual Case with a Review of the Literature
Article information
Abstract
Intraspinal teratoma is a rare subtype of spinal cord tumors, accounting for only 0.2% to 0.5% of cases. These tumors consist of a mixture of two or more germ cell layers, including the ectoderm, endoderm, and mesoderm. They often contain epithelial tissues, fatty tissues, and follicles derived from these three layers, which can be visualized on magnetic resonance imaging (MRI) in most cases. However, in our patient, a 35-year-old woman with an intradural oval intramedullary teratoma at the L4 level, the tumor exhibited features similar to those of a schwannoma. On MRI, the tumor appeared as a 2-cm mass attached to the end of the spinal cord, with intermediate signal intensity on T2-weighted images and iso-intensity without enhancement on T1-weighted images. L4 laminoplasty with tumor removal was performed under intraoperative monitoring. The tumor was found to be firm with a cyst containing mucoid and fatty tissue posteriorly. Near-total resection was achieved, although the tumor margin was indistinct. A literature review revealed spinal cord tethering and the presence of fatty tissue without MRI enhancement as characteristic findings of teratomas and dermoid cysts.
INTRODUCTION
Intraspinal teratoma is a rare subtype of spinal cord tumors, accounting for only 0.2% to 0.5% of cases. These tumors consist of a mixture of two or more germ cell layers, including ectoderm, endoderm, and mesoderm 20). Most cases of spinal cord teratoma occur in children, with only a few reported in adults 2). Teratomas are characterized by the presence of epithelial tissues, fatty tissues, and follicles derived from the three different germ layers. They can be confused with dermoid, epidermoid, and neurofibroma 1,24). However, in our case, the oval-shaped tumor exhibited an almost homogeneous signal intensity on T1- and T2-weighted magnetic resonance imaging (MRI) and appeared to mimic a schwannoma. In this paper, we will report an unusual case of adult intramedullary mature teratoma with a review of literature.
CASE REPORT
A 35-year-old woman visited our hospital complaining of waxing and waning nocturnal back pain. She also complained of a tingling sensation and numbness in both legs in the L4/5 dermatome without leg pain or bladder symptoms. MRI revealed a 2 cm-sized mass at the L4 level and accompanying spinal cord tethering with an intermediate signal in T2 and isosignal without enhancement in T1 images. Thin high signal intensity on the posterior of the tumor was found in T1 images, which was considered a fat signal. Computed tomography depicted an isodense mass without calcification (Fig. 1). No congenital anomaly was evident at the midline lesion of the patient. Based on these observations and the incidence rate of tumors in caudal lesions, we suspected atypical schwannoma, a dermoid cyst, or teratoma and planned L4 laminoplasty with tumor removal under intraoperative monitoring.

(A) Sagittal T2-weighted magnetic resonance image (MRI) showing an approximately 2-cm oval tumor at the L4 level. (B, C) T1-weighted MRI showing a thin hyperintense signal at the posterior aspect of the tumor, which was diminished on fat-suppressed images. (D) Slight enhancement at the lower poles was visualized using gadolinium diethylenetriaminepentaacetic acid. (E-G) Axial T2- and T1-weighted and enhanced MRI. The oval tumor showed relatively clear margins with the spinal cord and little enhancement. (H) Sagittal computed tomography revealing an isodense mass without calcification.
Midline durotomy exposed the tumor capsulated in a white fibrous sheath containing fat and fibrous tissues (Fig. 2A). The tumor was firm and immovable. Its posterior contained a cyst filled with mucoid and fatty tissues. At first, we recognized the pathway of nerves by stimulating the upper part and the lower part of the tumor. After stimulation, we found that the lower part of the tumor did not respond to the stimulation except for the upper part of the tumor (Fig. 2B). Further observation revealed anatomical features of the tumor and its degree of adhesion to adjacent structures and rootlets as well as its margin with the spinal cord. When we ruptured the cystic capsule to decrease the size of the tumor, some mucoid fluid spilled into the cystic capsule (Fig. 2C). We obtained a sample for frozen biopsy and attempted to enucleate the tumor center with an ultrasonic aspirator. The filum terminale was cut after confirming no response to the stimulation (Fig. 2D). The tumor at the spinal cord margin was removed while confirming the surgical plain by dissector (Fig. 2E). Finally, we achieved a near-total tumor resection, albeit with an indefinite tumor margin. Histologically, the tumor was composed of various types of epithelia, nerves, and adipose tissues consistent with mature teratoma (Fig. 3). The patient's symptoms were relieved after surgery. She was discharged about a week later without complications.
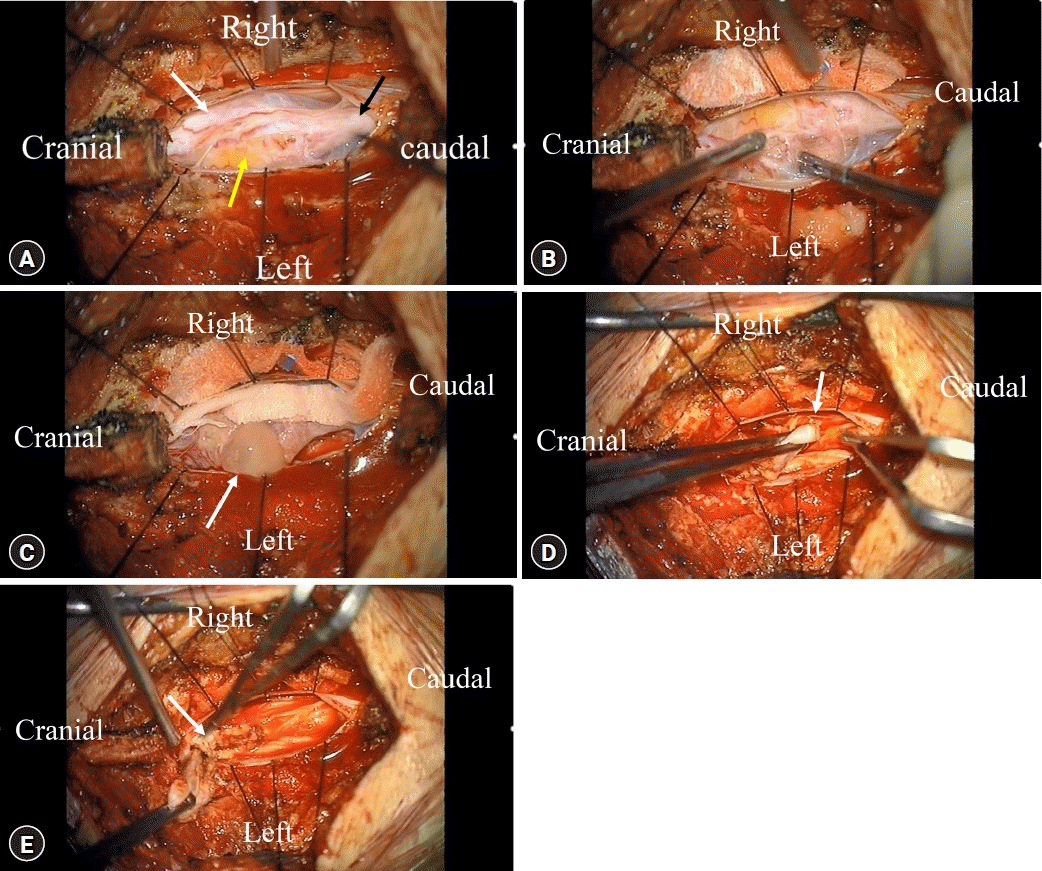
Microsurgical field of intramedullary teratoma in a caudal lesion. (A) Gross findings of the tumor after durotomy. White and black arrows indicate a tethered spinal cord and filum terminale, respectively. The yellow arrow designates the fat portion of the teratoma. (B) Stimulation was done to check the spinal cord and nerve pathways. (C) Central enucleation to decompress the tumor and rupture the mucus cyst. The white arrow indicates mucus fluid spilling out of the capsule. (D) Cutting the lower pole of the tumor. The white arrow indicates the ending of the filum terminale cut with a micro-scissor. (E) The remnant tumor was removed with micro-scissors. The white arrow indicates the surgical plane between the spinal cord and the teratoma.
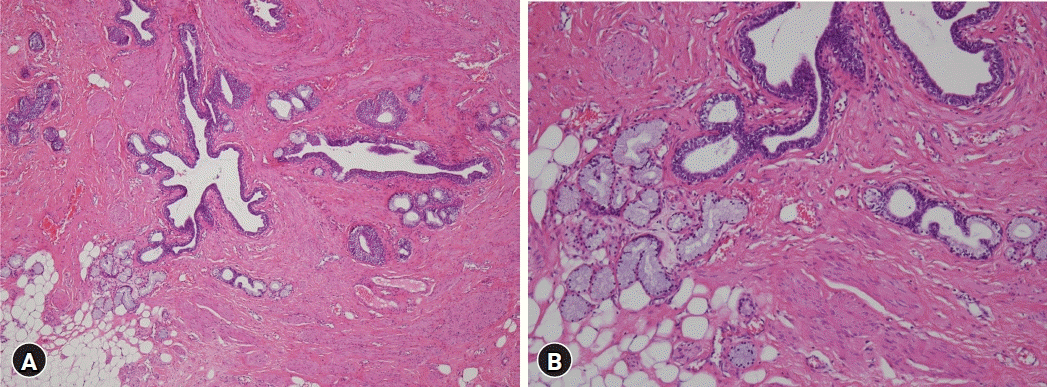
Histopathological analysis of the tumor tissue. (A) Photomicrograph showing a mixture of mature ectoderm, endoderm, and mesoderm. (B) Tumor components consisting of various types of epithelia, including mucous glands, nerves, and adipose tissues (A: hematoxylin and eosin [H & E] stain, ×40 magnification; B: H & E stain, ×100 magnification).
DISCUSSION
Teratoma is one of the rarest intraspinal neoplasms, tending to occur in young children, most commonly at midline sites. Excluding the sacrococcygeal variant, which is often encountered in neonates 9,24), they are rarely located in the spinal canal, with an incidence of only 0.2% to 0.5% of all spinal cord tumors 2). Previous adult IM teratoma cases in cauda equina lesions are summarized in Table 13-7,9-11,13,15,16,21-23,26,27,29. Our case is the 18th adult teratoma case reported so far.
Teratoma is described as a tumor that contains ectodermal, mesodermal, and endodermal components, originating early during embryogenesis when primordial germ cells from the yolk sac migrate aberrantly, usually into midline structures 3,18). Two theories exist regarding the origin of spinal teratomas. The first suggests that teratomas are due to misplaced pluripotent germ cells during cellular migration 14,17), while the second theory favors a dysembryogenic process as the origin of these tumors, suggesting that they ultimately arise from aberrant signaling during embryonic development 19,25). Teratomas are classically divided into three major subgroups: mature, immature, and malignant. Mature teratomas contain well-differentiated tissues from all three germ cell layers, while immature teratomas contain poorly differentiated, non-malignant tissues. Malignant teratomas typically are associated with York sac or endodermal sinus tissues with high levels of serum α-fetoprotein. These tumors are highly aggressive. They are associated with a poor prognosis 2,28).
The diagnosis of teratoma is accomplished by determination of tissues representing a mixture of all three germinal layers. Therefore, teratomas have several features distinguishing them from other tumors. First, they contain products derived from the three dermal layers such as fatty tissues, calcifications, epithelia, follicles, and glands. Second, they are usually accompanied by dysraphic congenital spinal malformations such as spina bifida and a tethered cord. Third, tumor enhancement is barely observed after gadolinium-diethylene triamine pentaacetic acid administration.
Tumor location, shape, and incidence are helpful features when diagnosing a tumor at a cauda equina lesion. Our case initially appeared to mimic a small oval schwannoma based on these characteristics. However, a review of described cases revealed several features of teratoma. A tethered spinal cord at the L3-4 level was observed in MRI. It could be one of the congenital spinal anomalies. A thin layer of hyperintense fat-like signal was observed at the tumor posterior in T1-weighted images. It may be due to differentiation of mesoderm. Difficult enhancement of the tumor in T1 images, one of the features of teratoma mixed with three dermal layers, was also observed. It was interesting that it had features of teratoma despite being a small tumor. We believe that these features are characteristics of spinal teratoma. Thus, they are diagnostically invaluable.
Surgical resection is a major tool for treating intraspinal teratomas, with standard microsurgical dissection such as laminectomy or laminotomy with tumor resection being the primary method of surgical treatment 8,12). The primary goal of surgery is to decompress neural elements without causing evitable damage to normal nerves. If possible, complete resection should be the goal of surgery. While total resection is the optimal goal, it is often infeasible due to tumor adherence to surrounding tissues. In our case, near-total resection was performed without any neurological deficits, although the surgical plan between teratoma and spinal cord was unclear.
CONCLUSION
Teratoma is one of the rarest intraspinal neoplasms. Surgery is the standard method for treating teratoma. Congenital anomalies such as a tethered spinal cord, high signal like fat, and difficult enhancement in T1 images are invaluable clues to diagnose and treat teratoma.
Notes
No potential conflict of interest relevant to this article was reported.
