Surgical Management of Recurrent Plexiform Neurofibroma in a Pediatric Patient with Severe Cervical Kyphotic Deformity: A Case Report
Article information
Abstract
Approximately 50% of patients with neurofibromatosis type 1 (NF1) develop orthopedic complications, and spinal deformities are common manifestations. Cervical kyphosis is comparatively rare in NF1; however, it results in spinal cord compression associated with paralysis and respiratory dysfunction, requiring surgical correction. Pediatric patients with NF1 usually have small and defective pedicles or lateral masses, and surgery with a single approach is limited to ensure sufficient spinal cord decompression and deformity correction. However, no reliable treatment guidelines are available for this challenging condition. This case report presents a 7-year-old patient with NF1 who had severe cervical kyphosis with intradural extramedullary neurofibromas. The tumors were removed before correcting the deformity to decompress the spinal cord and reduce the risk of spinal cord injury. Moreover, we effectively corrected and stabilized the kyphosis using the anterior-posterior-anterior approach.
INTRODUCTION
Neurofibromatosis (NF) is a disorder of the neural crest cells defined as a spectrum of multi-systemic diseases, primarily involving the skeleton, skin, and soft tissues. There are three types of NF: NF type I (NF1), peripheral NF, NF type II (NF2), central neurofibromatosis, and schwannomatosis. Neurofibromas are one of the most common manifestations of NF1, and especially among them, plexiform neurofibromas - arise from multiple nerve fascicles, branches, or plexuses involving connective tissue and skin folds10,14). They can grow to a large proportion, considered to be due to loss of NF1 gene expression in the intradural extramedullary distance, and leads to spinal cord compression14). Intra-spinal large tumors can destroy the vertebral structure and adjacent vertebral pedicle thinning, which can induce skeletal instability and compression of the spinal cord.
Orthopedic complications are mainly observed in NF1. Approximately 50% of patients with NF1 develop orthopedic complications, and spinal deformity is a common manifestation (15%-69%), including spinal kyphotic and scoliotic deformity1). Although cervical kyphosis is comparatively rare in NF111,16), it is characterized by acute angulation in the sagittal plane that results in life-threatening complications associated with paralysis and respiratory dysfunction that requires surgical correction. Before planning surgical treatment for kyphosis of NF1, understanding the curve pattern of the spinal deformity is essential. Dystrophic changes in NF1, illustrated as a short, sharp, and angular curve, include unstable pedicles, spindling of transverse processes, wedging of one or more vertebral bodies, and scalloping of the posterior, lateral, or anterior vertebrae1,7,8,16,18). These characteristics make it difficult to plan for its correction, and dystrophic curves should be treated aggressively with spinal fusion operation because of the strong tendency for curve progression18).
Several considerations are required to ensure sufficient spinal cord decompression and correction of the deformity in pediatric patients with cervical plexiform neurofibromas and severe kyphosis. For patients with severe cervical kyphosis, optimal angular correction with a single approach is limited, and surgeons need to consider surgery with a combined approach for effective tumor removal and correction of the deformity. However, there are no reliable treatment guidelines for this challenging condition, and several case reports have been published regarding various surgical treatments for the effective correction of severe cervical deformities in patients with NF1. A combined anterior and posterior approach can correct kyphosis by creating sufficient lordotic angles for spinal cord decompression with alignment correction. Although pediatric patients with NF1 associated with cervical kyphosis have much thinner, more fragile bone and more defective pedicles, we also need to consider a broader extent of fixation for stabilization, such as C1 or occiput–upper thoracic fixation.
We report the case of a 7-year-old patient with NF1 who underwent surgical excision of an intradural extramedullary plexiform neurofibroma due to a motor deficit caused by cervical cord compression. After 2 years, the recurrence of the tumor and aggravation of cervical kyphosis induced severe spinal cord compression. We performed tumor resection for cord decompression first and then planned surgical correction with three separate steps: anterior approach with corpectomy, posterior approach with the release of the fused posterior elements, and C1-thoracic fixation for kyphotic correction; and anterior support with mesh cage insertion. We effectively corrected and stabilized the kyphosis using the anterior-posterior-anterior (APA) approach.
CASE REPORT
A 4-year-old male diagnosed with NF1 when he was 2 years old visited the hospital with quadriplegia, unable to walk independently. In the cervical lateral simple radiograph, the cervical kyphotic angle was 18° (Fig. 1A). In the cervical spine magnetic resonance imaging (MRI), there were enhancing intradural extramedullary masses that originated from C5-6 nerve root, extended from the C2-6 levels, and compressed the posterolateral spinal cord (Fig. 1B). To decompress the spinal cord, we needed to remove the tumor and correct the vertebral curvature. Considering the higher risk of iatrogenic cervical kyphosis in children, we suggest the laminectomy with posterior fixation, however, because the pedicles and lateral masses of the cervical spine were too small to insert screws for kyphosis correction (Fig. 1C). We decided to perform a laminoplasty of the C3, C6 and a total laminectomy of the C2, C4, C5 with tumor removal for rapid spinal cord decompression to prevent the progression of neurologic deficit and increase the possibility of recovery. We performed subtotal resection of the tumor except residual tumor adhered to C6 nerve root. The tumor showed histology of plexiform neurofibromas without malignant transformation. On postoperative cervical spine MRI, spinal cord was fully decompressed and residual tumor was remained on C6 root. The patient’s neurological function improved after surgical resection and rehabilitation. Finally, he could walk with help for approximately 10 min, go upstairs, and play with toys independently.

(A) Initial lateral cervical spine radiograph when the patient was 4 years old. The radiograph in the neutral position shows classic cervical kyphosis with neurofibromatosis type 1. (B) Preoperative coronal and sagittal magnetic resonance imaging showing the enhancing tumor origin from C5 and C6 nerve roots extending from C2 to C6. Axial imaging demonstrating that the tumor compresses the cervical cord postero-laterally. (C) Axial image of cervical spine computed tomography shows small lateral masses and pedicles from C1 to T1.
According to the possibility of deteriorating cervical deformity and the tendency of relapse of plexiform neurofibroma in patients who are younger than 10 years at the time of surgery12) with non-extremity lesions, or who has residual tumors, we evaluated him every one or two months with cervical radiograph or computed tomography (CT), and he had been receiving treatment for NF1 with targeted therapy. For managing tumor progression and correcting deteriorating cervical kyphosis, we considered early re-operation, but it was delayed because of personal problem of him and his family. Cervical kyphotic deformity progressed through two years, with gradual aggravation of motor deficits. At his last visit before surgery, the patient could not walk without help and lost strength in the upper extremities; the patient could not lift a spoon to eat anything independently. A series of cervical lateral radiographs for 2 years, showed that the kyphotic angle was aggravated from 45° to 85° (Fig. 2). We followed up the cervical spine MRI (Fig. 3A), in which an extensive recurrent tumor combined with progressive cervical kyphosis led to severe spinal cord compression. Three-dimensional CT showed bone fusion of the C2/3 facet joint (Fig. 3B). As the patient grew up, the pedicles and lateral masses of the C1-2 became sufficiently large to insert screws for kyphosis correction (Fig. 3C). Finally, His parents decided to perform surgery to correct the kyphosis and remove the tumor for sufficient decompression.

A series of lateral cervical spine radiographs for the 24-month follow-up. (A) The immediate postoperative sagittal Cobb angle of kyphosis was 45°. (B-E) As time passed, the sagittal Cobb angle increased from 50° to 85° and cervical kyphosis worsened.

(A) Two years postoperatively, when the patient was 6 years old, a follow-up sagittal view of cervical spine magnetic resonance image showed severe cord compression due to recurrence of neurofibroma with progressive cervical kyphosis. (B) Preoperative computed tomography demonstrating the fused C2-3 facet joint (red arrowhead). (C) Axial image of cervical spine computed tomography showing that both lateral masses of C1 and both pedicles of C2 had become large enough for screw insertion.
In our detailed surgical planning, considering the patient's very severe kyphosis, a single anterior or posterior approach is not sufficient, and a combined approach, especially a 3-stage, is needed. In the anterior approach, kyphosis is corrected by removing the dystrophic vertebra and grafting it through anterior lengthening. In the posterior approach, kyphosis is corrected by releasing the fused mass and performing posterior shortening with compression through strong fixation.
Tumor Resection
In the initial operation, we performed a C2-6 total laminectomy and incision of the dura. The ventral and dorsal portion of the tumor was adhesive to the spinal cord and C6 nerve root, and we performed gross total resection of the intra-dural tumor from spinal cord with meticulous dissection. No significant changes in intra-operative monitoring were observed. The patient’s immediate postoperative motor power improved to grade 3. Neurological and respiratory function gradually worsened in 2 days, postoperative spinal MRI showed T2 hyperintensity in the cord suggesting spinal cord edema with tiny residual foraminal tumor on C6 nerve root and the patient followed up with steroid therapy and rehabilitation.
Deformity Correction
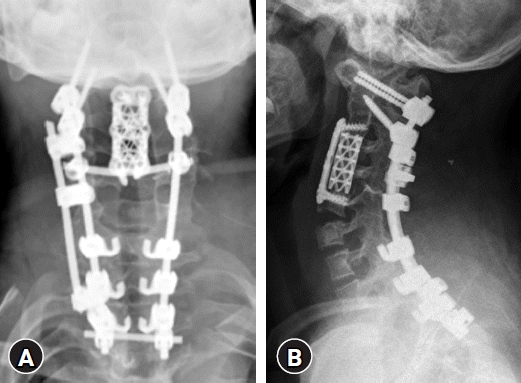
Anterior (corpectomy)-Posterior (lateral mass, pedicle screw fixation and kyphosis correction)-Anterior (anterior vertebral column reconstruction) approach (Fig. 4)
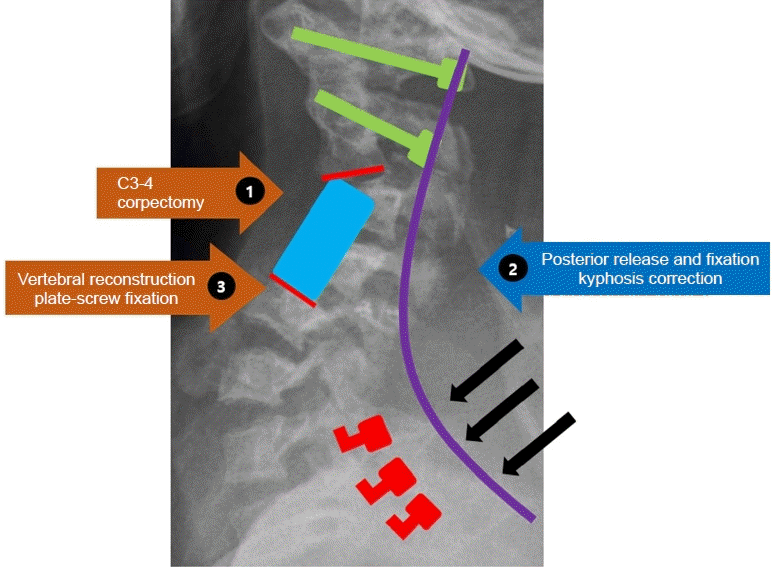
Simulation of the anterior-posterior-anterior approach we performed, involving C3-4 corpectomy with prior application of screws for both lateral masses at C1 and both pedicles at C2, with contoured rod and kyphosis correction by compressing the rod. Finally, anterior vertebral reconstruction was performed, with mesh and plate screw fixation.
After 2 weeks, the patient’s postoperative neurologic deficit was getting better, we decided to continue with the second operation. In the first stage, the anterior approach, we performed C3, 4 corpectomy without mesh cage insertion to reduce spatial limitation that could be created by anterior fixation and to make a sufficient angular correction when performing kyphosis correction via posterior fixation. In the second stage, changing the operation position from supine to prone, a total laminectomy from C2-6 was performed to depress the spinal cord and a facetectomy to release the inflexibility of fused C2/3 facet. Next, we inserted a lateral mass screw and pedicle screw in C1 and C2 respectively; applied hooks to C7, T1, and T2 instead of using a pedicle screw. We placed a contoured rod which is adjust to natural cervical curvature. First, contoured rods are locked on both C1 and C2 screws and we lifted upper cervical bodies by compressing the rods and locking the caps with hooks of C7, T1, and T2. We implemented intraoperative neuromonitoring frequently during posterior correction with contoured rod fixation because of the extreme flexibility before cage insertion and anterior fixation were performed. In the third stage, the operation position was changed to supine, and a mesh cage was inserted. The plate was applied at C2-5 for anterior fusion.
The kyphosis was corrected to 29° on a simple lateral radiograph, and his motor power improved (3 to 4/5 strength of the upper extremities and 2/5 strength of the lower extremities). Two years after the surgery, bone fusion had successfully progressed (Fig. 5). However, the patient still suffered from gait disturbances because of spasticity. He is still treated by target therapy at another center, on follow-up spinal MRI at 18 months after final surgery, there is no evidence of recurrent tumors.
DISCUSSION
Because of extreme small and weak lateral masses and pedicles of cervical vertebra that were not suitable to perform screw fixation in pediatric patients, the authors suggest three different plans. First, after performing an immediate decompression of spinal cord by laminectomy or/and laminoplasty, as in this case, it is to observe the development of neurologic deficit or pain while the bones of children grow to appropriate size to insert devices. Secondary, corpectomy with mesh cage or bone graft and mini-plate with screw fixation could be performed to achieve anterior support preventing progression of cervical kyphosis and spinal cord compression. Following anterior cervical corpectomy and fusion, application of halo-vast is needed to provide the additional support. However, infection via halo-vast is fatal to children and there is a risk of weakness of neck muscles. In addition, occipito-thoracic fusion could be considered for fixation point instead of cervical lateral masses and pedicles, but limitation of neck movement could be also considered.
There are several factors causing the progressive cervical kyphosis in this patient. First, the preexisting cervical kyphosis could lead to progressive cervical kyphosis with the demyelination of nerve fibers in the funiculi, ultimately leading to neuronal loss, repeated mechanical compression, and ischemic damage in the anterior horn in a vicious cycle15). In addition, the inherent weak neck muscles in pediatric patients and dystrophic change of the vertebral body in NF1 also contributed to cervical kyphosis. Above all, preceding laminoplasty or laminectomy could accelerate cervical kyphosis with injury of the posterior tension band and increase the compressive burden of the anterior column of the spine. Especially in our case, neurofibromatosis commonly develops the deformity of vertebral body such as a wedge body as a natural course that might contribute to aggravate cervical kyphosis. And there is a higher incidence of post-laminectomy kyphosis in children because of higher viscoelasticity of the ligament of them13,21). Yasuoka et al.21) recommended follow-up by repeated radiographs, early anterior fusion, and immobilization with a brace to prevent post-laminectomy cervical kyphosis.
To sufficiently decompress severe cervical spinal cord compression caused by plexiform neurofibroma and severe cervical kyphosis, we considered 4 factors that could complicate the re-operation. (1) Which should be performed first between tumor removal and correction of the kyphosis; (2) single or combined approach; (3) direction of kyphosis correction; (4) fixation extents and methods.
At first, we decided to perform both tumor resection and deformity at separate times for several reasons. Resection of the tumor and additional spinal cord decompression with large angle correction of cervical kyphotic alignment at the same time could take too long time of surgery and increase the risk of postoperative neurologic deterioration. It cannot be explained by any other causes but by reperfusion injury after excessive spinal cord decompression, so-called white cord syndrome3). In theory, acute cord decompression with expansion of chronically ischemic cord could alert perfusion due to recoil of the spinal architecture. In rats, Yang et al.20) proved free radicals induce direct cellular injury to spinal cord axons through oxidative stress-triggered cascade of reperfusion. In addition, we suggest the greater correction made by tumor resection with deformity correction could induce greater distraction under axial strain that allows the deterioration of neurologic function5). When kyphosis correction is performed before tumor resection, we judged that there was a risk of severe spinal cord injury because the space-occupying plexiform neurofibroma restricted the correction of kyphosis and mechanical spinal cord compression became more severe; hence, tumor removal was performed first as described by Kawabata et al.7).
There are a lot of case reports that prove necessity of a combine approach for the correction of severe cervical deformity with successful fusion. Sirois and Drennan16) reported a 72% incidence of failure when patients with dystrophic kyphotic curves of ≥50° underwent posterior fusion alone. They also reported that the incidence of pseudoarthrosis was significantly higher in the isolated posterior fusion group (38%) because the dystrophic changes in the laminae and lateral masses were too severe to allow solid bone union by posterior fusion alone. Vadier et al.19) reported on a 13-year-old patient who had a cervical kyphotic deformity due to neurofibromatosis, and the deformity was corrected from 82° preoperatively to 18° postoperatively using combined anterior-posterior spinal arthrodesis.
Surgical planning is determined by many factors, such as neurologic status, presence of neural compression, etiology of the deformity, flexibility, presence of the ankyloses, and/or fusion mass13). In general, important points in decision-making for cervical kyphotic deformity correction are the flexibility of the deformity and appropriate surgical approach for deformity correction. In the case of ankylosed fixed deformity, effective correction is possible only when the fusion site is released. Therefore, the surgical approach varies depending on where the ankylosed or fused site is located6).
Our case is an NF1 patient with severe dystrophic changes, severe cervical kyphotic deformity of 85°, and the fusion state of the facet joint of C2-3. Although the exact cervical alignment parameters in children have not been validated and the amount of cervical kyphosis correction has not been determined, we planned to create a straight spine at least as reported by Stenmets et al.17) to achieve effective spinal cord decompression through sufficient kyphosis correction. Etame et al.4) reported that ventral release and fusion had the least amount of correction of 11° to 32°, while dorsal pedicle subtraction osteotomy had 23° to 54°, which was similar to the ventral and dorsal combined approach that provided a range of 24° to 61.4° in Cobb angle. The surgical technique commonly used to correct the deformities consists of correction and stabilization of the deformity and decompression of the neural elements, requiring carefully coordinated execution of anterior column lengthening and posterior column shortening.
In our detailed surgical planning, considering the patient's very severe cervical kyphosis, a combined approach was required. In the anterior approach, kyphosis was corrected by removing the dystrophic vertebra and grafting it through anterior lengthening, while the posterior approach, corrected kyphosis by releasing the fused mass and performing posterior shortening with compression through strong fixation.
Posterior release of fusion mass should be performed before anterior lengthening in the principle of general cervical kyphosis correction2), however, if the release of facet is performed before the decompression of the anterior column, there is a high possibility of exacerbating myelopathy due to spinal cord stretching and ischemia. In addition, owing to the use of graft materials (such as mesh cage or allograft) for vertebral reconstruction after corpectomy of dystrophic vertebrae during the anterior approach after the posterior approach, kyphosis correction by compression through the third posterior approach could be limited. End plate damage may occur during the stage of posterior shortening by compressing the anterior graft. Therefore, we planned an A-P-A combined approach.
Another considering point is fixation methods and extent. Due to the lack of adequate and especially designed pediatric spine instrumentation and small-sized vertebra, instrumentation for deformity correction in the infantile and pediatric cervical spine is challenging. Surgical treatment can be complicated by inadequate and less rigid instruments that can lead to progressed deformity and neurological deterioration due to pseudarthrosis and loss of correction.
According to Kokubun et al.9), the mean age of patients with cervical kyphosis and NF1 at diagnosis was 20 (1–38) years. The mean age of patients was 19 years at surgery in this study. Therefore, a well-defined strategy for pediatric patients with severe cervical kyphosis and NF has not been developed. Pediatric patients with NF1 have small dystrophic pedicles, lateral masses, and vertebral bodies where stable anchors can be placed. This case is a 6-year-old patient with a diminutive and dystrophic pedicle and lateral mass, and fixation points were insufficient and inappropriate, making firm fixation for kyphosis correction challenging. As the size of the pedicle and lateral masses from C3-C7 was small, we judged that instrumentation was impossible. Therefore, lateral mass and pedicle screw fixation were performed on C1 and C2 separately, which were relatively suitable for fixation, and lamina hook fixation was planned on C7, T1 and T2.
CONCLUSION
The surgical approach for severe cervical kyphosis with intradural extradural tumor in pediatric patients with NF1 is challenging and complicated because of the risk of postoperative spinal cord injury, the poor bone fusion rates, and too small lateral masses and pedicles to insert screws and too vulnerable to bear the posterior shortening force created by posterior fixation. We successfully performed combined A-P-A surgery on pediatric NF1 patients with severe cervical kyphosis and achieved spinal cord decompression and effective kyphosis correction without surgical complications. This surgical strategy could be another option for severe cervical kyphosis in pediatric patients.
Notes
No potential conflict of interest relevant to this article was reported.