Intradural Spinal Cysticercosis: Case Series
Article information
Abstract
Objective
Literature concerning treatment with focus on case presentation, mostly, consists of case reports. In this report, we will present the characteristics of the disease and results of seven cases of spinal Neurocysticercosis (NCC) patients.
Methods
We retrospectively searched data for eligible patients between 1998 and 2014. Total of seven patients were enrolled, and it referred from pathologic result only. Medical records and images were reviewed for preoperative and postoperative clinical, radiological, and pathological results. All patients were treated with surgical treatment with antibiotics and followed up for 43.1±10.23 months (range, 13–98 months).
Results
Among seven cases, the pathologic lesion was located in intradural-extramedullary (IDEM) portion in six cases, one case was found in intramedullary (IM) portion. Only 28% (2/7) showed brain involvement with spinal cysticercosis. Preoperative symptoms consisted of motor deficit were found in 57% (4/7), sensory change in 43% (3/7), gait ataxia in 43% (3/7), neurogenic bladder dysfunction in 29% (2/7). With regard to pain syndromes, all patients presented pain, including leg pain in 71% (5/7), and back pain in 43% (3/7). At final follow up, neurologic status after treatment showed complete recovery for 43% (3/7), improvement for 43% (3/7), and unchanged status for 14% (1/7).
Conclusion
Surgical removal of pathogen showed clinically successful results, therefore early operative treatment should be considered in case of neurologic deficit even it is intradural lesion. Twenty eight percent among the patients with spinal cysticercosis showed concomitant brain lesion, thus, brain exploration should be needed at the time of diagnosis.
INTRODUCTION
Neurocysticercosis, an infection caused by Taenia solium larvae, is the most common parasitic infection of the central nervous system (CNS). Cysticercosis is an endemic in low-income and lower middle-income countries’, mainly affecting Africa, Latin America, and Asia8). In endemic villages in Latin America, the seroprevalence has been estimated to be between 4.9% and 24%23). Using combined diagnostic procedures, such as ELISA (enzyme-linked immunosorbent assay), brain computerized tomography (CT) or brain magnetic resonance imaging (MRI), 82 neurocysticercosis cases were detected in a university during a period of 3–4 years in the early 1990s in Korea4).
Intracranial involvement is frequent, whereas spinal involvement of NCC is rare and accounts for 0.7% to 1% of CNS cysticercosis1,12,24). There are four difference types of spinal cysticercosis: extradural, intradural, subarachnoid, and intramedullary. About 80% of spinal cysticercosis are intradural-extramedullary8). Intramedullary spinal involvement is the rarest manifestation of NCC.
The clinical presentation depends on the stage, number, size and location of the cysts within the CNS, and neurocysticercosis is more serious than musculoskeletal or subcutaneous cysticercosis. In brain cysticercosis, seizures occur in 50% to 80% of patients with parenchymal disease10,18). Spinal cysticercosis may be present with radiculopathy or myelopathy including neurologic deficit such as motor weakness and bladder dysfunction. Only a few cases have been published, and to the best of our knowledge, only few articles were reported about detailed surgical outcome in spinal intradural NCC. Literature concerning treatment with focus on case presentation, mostly, consists of case reports and small patient series. This study was designed to present the clinical characteristics and surgical results of seven cases of spinal NCC patients who were treated in a single institute.
MATERIALS AND METHODS
All surgically treated patients in our institute were prospectively enrolled in our documentation system. To conduct this study, we retrospectively searched this data base for eligible patients between 1998 and 2014. Patients are selected by diagnosis as a cysticercosis, and it referred from pathologic result after surgical removal and serologic test. A total of 9 patients were diagnosed as spinal cysticercosis, however, in two of nine patients, there were no sufficient medical charts with radiologic images to study. Thus, we decided to exclude these two patients and finally enrolled seven patients. Operative procedures were performed by five senior neurosurgeons within a standardized surgical setting. Pre and immediate post-operative MRI included routine sagittal T1-weighted sequence without and with contrast as well as axial and sagittal T2-weighted sequence. Surgical approaches were chosen based on the cyst as well as the overlying parenchyma layer. Goal of surgery was to create a large enough fenestration with total cyst removal using optimized minimal bony approach. All operation was performed under multimodal electrophysiological monitoring consisting of somatosensory-evoked potential (SSEP) monitoring, motor-evoked (MEP) monitoring. For clinical evaluations, patients were routinely seen at follow-up visits in our outpatient clinic within the first month and sixth month after operation. Neurological outcome was extracted by reviewing patients’ medical charts and electronic documentation at follow-up visits. Pre, immediate postoperative and long-term MRI scan were performed and evaluated by an experienced neuro-radiologist.
For evaluating clinical results after surgery, pain can be different by lesion level, thereby categorized by one category, and other category are selected by important and common neurologic deficits in spine disease such as motor deficit, sensory deficit, gait ataxia, and neurologic bladder dysfunction. Overall outcome is determined on the basis of the symptoms which showed less recovery.
RESULTS
Seven eligible patients for this study were identified. Five patients were male, the other two patients were female, and the median age of the patients was 50 years (range 30–70 years). In six cases, the lesion was located in the IDEM area, in the remaining case the lesion was found within the IM area. The lesions appeared at a similar rate in the thoracic and lumbar level, although there was no lesion on the cervical level. Only 28.4% (2/7) showed brain involvement with spinal cysticercosis. The baseline patients’ characteristics are demonstrated in Table 1.
All seven patients showed neurological deficits and/or pain syndrome. Preoperative symptoms consisted of motor deficits were found in 57%(4/7), sensory change in 43%(3/7), gait ataxia in 43%(3/7), and neurogenic bladder dysfunction in 29%(2/7). Associated with pain syndromes, all patients presented pain, consisted of leg pain in 71%(5/7), and back pain in 43%(3/7).
At the last follow-up no patients suffered from newly developed neurologic symptoms or aggravation of preoperative symptoms. The overall neurological outcome analyses showed complete remission for 43%(3/7), improvement for 43%(3/7), and unchanged status for 14% (1/7). In terms of pain syndrome, All patients revealed complete remission (7/7, Table 2). With regard to motor deficits of 4 patients, three patients (75%) showed improvement compare to preoperative status. One patient had weakness on the right big toe (Grade IV) preoperatively which was unchanged postoperatively. Among two patients (29%) with neurogenic bladder dysfunction, one patient (50%) showed complete remission, and one patient (50%) showed improvement and was able to urinate by himself. The detailed outcome data is shown in Table 2.
Case Illustration
1. Patient 1
A 70-year-old male patient was admitted due to right leg pain with weakness of right ankle dorsiflexion (Grade III) with numbness. On radiological evaluation, Preoperative spinal MRI showed a multiple intradural-extramedullary (IDEM) cystic mass from the T1 to 10, L1 to 3 levels and cord edema from the T1 to T10 level.
The patients underwent the operation, which involved extensive laminectomy to remove the cystic mass and relieve spinal cord compression. Under the microscope after opening of the dura, a firm cystic mass was found with a well demarcated margin and removed totally without damage. The surgical pathology from the T1 to 10, L1 to 3 levels was determined to be a parasite consistent with cysticercosis.
We performed a brain MRI and it revealed no evidence of cysticercosis. After the surgery, his leg pain and numbness were slowly improved. A postoperative MRI confirmed no remnant pathology. The patient was discharged two weeks after surgery and took albendazole for 4 weeks. At the final follow up visit, His pain had mostly gone, and his right ankle dorsiflexion weakness had improved from Grain III to Grade IV.
2. Patient 7
A 45-year-old male patient was admitted via the outpatient department due to a headache, and diagnosed as a brain cysticercosis. Praziquantel twice a week and albendazole 3 times a day for 8 days were used because of recurrence of cysticercosis.
Four years later after complete remission of brain cysticercosis, the patient visited again spine center due to progressive weakness and pain in his right leg, which had been sustained for a week. His hip and leg flexion and extension were grade II and couldn’t ambulate independently. On radiologic examination, Whole spine T2 weighted MRI revealed large amounts of cystic lesion in the upper thoracic spinal canal and diffuse high signal intensity which are suggestive of cord edema below the lesion. A Multi-lobulated cyst was found on the posterior portion of the spinal cord from the T11 to L2 level. Brain MRI showed the CSF collection on the posterior to cervicomedullary junction, probably associated with inflammation, and no more evidence of active cysticercosis was confirmed.
The patient underwent a total laminectomy from T1–2, T11–12, and L1–2 to decompress the spinal cord. The spinal cord was severely compressed both in the upper and lower thoracic area and diffuse arachnoiditis was observed. After the laminectomy, cord compression was released and the flow of CSF was improved. After the operation, the patient started rehabilitation treatment, and his motor grade was improved. Initial motor was grade II, after rehabilitation period, it improved until grade IV and the patient could ambulate with a cane.
Six months later, a patient came to an outpatient clinic with left leg weakness and hesitancy. He presented weakness on both hip flexion, extension (Grade II) and knee and ankle (Grade III). We scanned a spine MRI that revealed that a cystic loculation of CSF in the upper thoracic spinal canal had increased and that no significant interval change of leptomeningeal enhancement at the cervico-medullary junction was present. We diagnosed it as a cysticercosis recurrence, and decided to perform an operation.
We did a total laminectomy on the T3–4 level, and removed the cystic mass with adhesiolysis. During the operation, we removed the cystic mass with lesions like larvae, and the pathologic result was cysticercosis. After laminectomy, the flow of CSF improved. We started praziquantel for 3 weeks, and also started rehabilitation treatment.
One year later after operation at the outpatient clinic, we checked a follow-up MRI and it revealed that the cystic loculation of CSF had increased in the upper thoracic spinal canal and another fluid collection was found on the thoracic T10 to 11 levels. The diffuse thoracic cord edema on T2 weighted MRI was significantly decreased. Newly developed symptoms were not found, and the previous patient’s motor weakness of both legs had improved to grade IV so that he can now walk with a walker. Urinary symptoms also improved and he can now urinate by himself without intermittent catheterization.
DISCUSSION
Cysticercosis, the most common parasite disease, is caused when Taenia solium invades the central nervous system1). The pig is the intermediate host and humans are generally the definite host, although sometimes the human becomes the intermediate host by chance1,13,14). CNS involvement of the neurocysticercosis is mostly in the brain and spinal involvement is very rare24). Most authors reported an incidence of spinal neurocysticercosis to be of 0.7% to 1% of CNS cysticercosis1,12,24).
Involvement of the CNS is about 60% to 90% of patient of all cysticercosis infection16,17). Brain parenchyma is most likely seeded through hematogenous dissemination2,7). Spinal cysticercosis most commonly involves the subarachnoid space by the extension of cerebral subarachnoid disease, but intramedullary may also occur hematogenously24).
Spinal cysticercosis most commonly involves the subarachnoid space and results in intradural cysts or arachnoiditis. The symptom of spinal cysticercosis may vary depending on the size and location of the lesion1). One of the most important pathophysiological mechanisms is direct mass effect, myelopathy from cord compression3). Parasite metabolism or cyst degeneration may also make an inflammatory reaction which makes symptoms. Vascular insufficiency can cause symptoms including meningitis or cord degeneration21).
This study represents a detailed clinical follow-up of seven patients undergoing operation of cysticercosis. Based on our patients’ outcomes, surgical treatment for symptomatic spinal intradural cysticercosis is safe and effective for adult patients with progressive neurologic deficit. All patients were treated within a standardized setting including preoperative MRI and electrophysiological monitoring. Not all patients were scanned for the immediate postoperative MRI, but they were scanned a few months later at an outpatient’s clinic. All the patients who were admitted had different symptoms including pain, motor deficit, sensory change, and bladder dysfunction. Pain was observed in all patients, and most common symptom was leg pain. Motor weakness and sensory change were also found at a higher rate of 57% and 43%. The ratio of motor weakness was higher than intradural spinal lesion like benign tumor. Reviewing our neurological outcome analysis, motor deficits and pain syndrome showed the best overall recovery, considering the limitation of a small sample size.
The duration of medical treatments for cysticercosis is unclear. In some reports, cure was achieved with 2 weeks of therapy10), whereas others suggested 4 to 8 weeks of therapy12). Our paper presents recurrence in two patients who took medication for 1 week and 2 weeks each. In patients without recurrence, they use medication for more than at least 4 weeks. Some studies show that the treatment was equally effective whether given medication for seven days or 14 days11). Rather, the period of taking medications had relationship with recurrence. Although there is a possibility of adverse effects, we suggest to use enough period of taking medicine to reduce the rate of recurrence. Besides, some studies show that albendazole is more effective than praziquantel, even both albendazole and praziquantel are effective to NCC9).
In our study, all patients underwent operation. Most of the patients underwent laminectomy and cyst removal, but not all patients. Two patients underwent only laminectomy but they showed an improvement of their preoperative symptoms. A successful clinical results for associated pain syndrome has also been described by other authors6,12). Especially about patients with motor weakness, we found severe cord and root compression due to cyst and arachnoiditis intraoperatively. And after laminectomy, severe compression of the cord and root was released and patients showed improvement of weakness. Neurologic deficits due to cysticercosis in this series tend to be relatively reversible than other intradural pathology such as tumor, inflammation, and traumatic injury. In spinal NCC, outcomes in patients are related to the following factors: location, the severity of inflammation, and chronicity of symptoms/time to treatment. Acute neurological deterioration is mostly related with mass effect, and is often resolved after prompt excision of the lesion1). Several authors have indicated that patients who suffer from rapid subarachnoid cyst induced deterioration may improve and return to full activity, but nearly 50% experience some continued or recurrent symptoms attributed to arachnoidal inflammation5,19). Thus based on both previous reports and our clinical case series, patients with neurologic deterioration could be considered for early surgical intervention.
Nevertheless, surgery may not be necessary in all cases. Some case reports showed that use of medication only can improve neurologic symptoms including motor weakness6,12). In general, the operation proceeds, but it is debatable yet. As we have seen a good postoperative prognosis in this paper, we suggest to surgical intervention with decompression and removal of pathology if possible.
MRI is the imaging study of choice, because it is more sensitive than CT for all phases of the disease process. The intradural extramedullary involvement was represented by cystic structures within the spinal subarachnoid space or by homogeneous sheet like enhancement of the perimedullary spinal subarachnoid space after gadolium administration. The patients with intramedullary involvement showed either focal cystic lesions within the parenchyma of the spinal cord or multisegmental syrinx formation apparently related to the preceding chronic cysticercotic arachnoiditis1,13). Occasionally the actual scolex is visualized on MR images as a mural nodules1,13). In our paper, most of cases showed cysts in subarachnoid space and arachnoid adhesion with rim-enhancement after gadolium administration. Spinal cysticercosis can be easily misdiagnosed as an arachnoid cyst, and these lesions are usually indistinguishable without ELISA and contrast-enhanced MRI thus, Contrast-enhanced MRI is mandatory for evaluating spinal cysticercosis22). The wall of the arachnoid cyst does not appear enhanced, whereas that of the cysticerci are well enhanced in gadolinium enhanced MR images20).
MRI of patient with intramedullary involvement showed cyst on T2-weighted sagittal images at intramedullary location with partially arachnoid adhesion without any evidence of actual scolex. Most of neuroimaging findings of cysticercosis did not showed pathognomonic finding. If spinal cysts are shown in spinal MR, consideration of cysticercosis should be needed based on clinical, radiological, immunological, and epidemiological data.
Spinal NCC occurs in patients with an established diagnosis of intracranial NCC in approximately 75 % of the cases, and isolated cases of spinal NCC are thought to be uncommon5,15). In our paper, 40% (2/5) of cases showed brain involvement with spinal cysticercosis. Due to the high relevance of the brain and spinal cord lesion, a brain exploration should be needed in spinal cysticercosis.
The comparison of single and multiple lesions can be a one of major concern. Our data had one patient (14%) with single lesion, had only pain without weakness. It may differ from spine, in brain NCC cases, cases with multiple cysts may have more clinical manifestation such as epilepsy, focal deficits and more change to show arachnoiditis, encephalitis, even hematoma25). Because of not enough research for comparison of single and multiple lesions for spinal NCC, additional research will be needed.
CONCLUSION
Spinal neurocysticercosis is rare and incidence shows gradually declining trend. Nevertheless, we have to discriminate all cyst-related diseases occurred in spine. Surgical decompression and removal of pathogen showed clinically successful outcome, thus early operative treatment should be considered in case of progressive neurologic deficit especially in intradural extramedullary lesion. Brain exploration should be performed in every case due to relatively high rate of concomitance. And we suggest the medication maintenance more than four weeks might be important to reduce recurrence rate.

First patient’s preoperative T2-weighted MRI (A) revealed loculated cystic lesion in intradural-extramedullary area at ventral aspect of spinal cord at T1 to 10 and L1 to 3 levels. Especially gray matter edema is shown at C7 to T1 level. Second patient’s preoperative T2-weighted MRI (B) revealed multi-lobulated intradural cystic mass at L4 to S1 level.
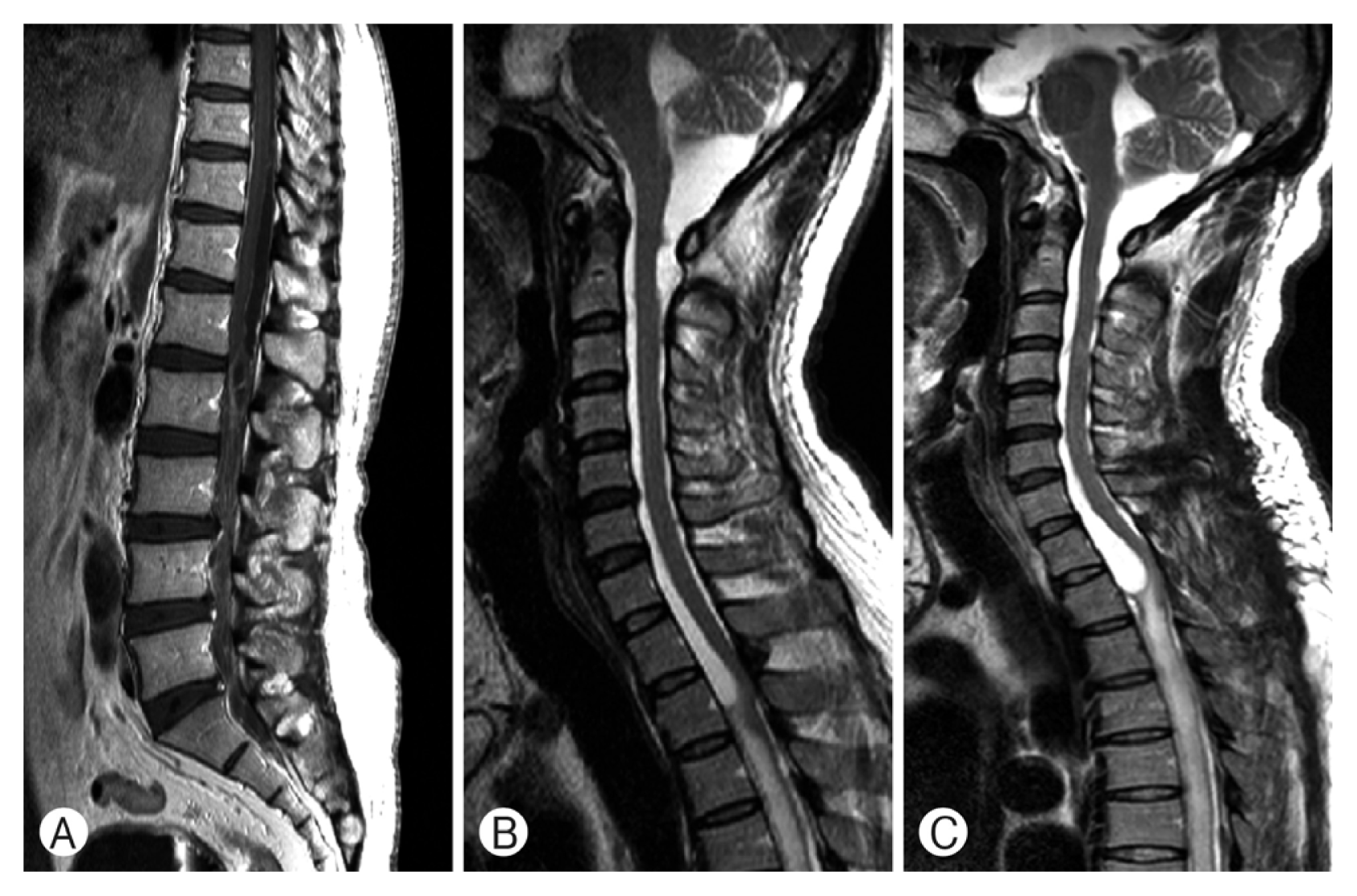
Seventh patient’s preoperative T1-weighted lumbar MRI (A) revealed loculated cystic lesion in intradural extramedullary area at T11 to L2 levels. T2-weighted cervico-thoracic MRI (B) revealed diffuse arachnoiditis with CSF collection at T1 to 5 level. Postoperative T2-weighted MRI (C) of first operation showed increased amount of cystic loculation of CSF and diffuse myelopathy at upper thoracic level.