Treatment Outcome of Gamma Knife Radiosurgery of Vestibular Schwannomas with Cystic Component
Article information
Abstract
Objective
Many studies have shown favorable outcome of Gamma knife radiosurgery (GKRS) for vestibular schwannomas (VSs). However, there have been few studies which have shown the treatment result of cystic VSs (CVSs) for GKRS. The aim of this study was to confirm whether GKRS for CVSs continues to be safe and effective.
Methods
The study population consisted 511 patients (172 males, 339 females) with VSs treated with GKRS between May 1990 and December 2013. Group 1 defined as cyst/tumor volume >30%, group 2 was cyst/tumor volume >50%, and group 3 was solid tumor or cyst/tumor volume ≤30%. To analyze factors that correlated with treatment outcomes, the following factors were assessed: tumor volume, cyst volume, tumor/cyst ratio, cyst type (single vs. multicystic), previous resection history, marginal radiation dose.
Results
The median follow up period was 52 months (range, 12–110 months). Tumor progression in group 1 was 5 (18.5%), group 2 was 1 (11.1%) and group 3 was 31 (6.4%). In group 1, 2 patients underwent delayed microsurgery at 31 and 36 months after GKRS.
Conclusion
In this study we found that GKRS for large CVS was associated with poor treatment outcome. Further study is needed to identify the mechanism of the tumor progression of CVS after GKRS
INTRODUCTION
Vestibular schwannomas (VSs) are relatively common intracranial tumors, account for 10% of all primary intracranial tumors13), are the most common tumors occupying the cerebellopontine angle, and accounts for 80% of cerebellopontine angle tumors. Numerous studies have reported incidence rates ranging from 0.2 to 1.7 per 100,000 population7).
Based on their consistency, they can be broadly grouped into cystic VSs (CVSs) and solid VSs (SVSs)12). CVSs are believed by some to have a more aggressive course with more rapid neurological deterioration and have less favorable surgical outcomes than SVSs with regard to facial nerve outcomes, surgery-related complications, and mortality1,3).
It is widely believed that CVSs have less favorable surgical outcomes than SVSs with regard to facial nerve outcomes, surgery-related complications12). And the Marseille group reported treatment failure rate of CVS treated with Gamma knife radiosurgery (GKRS), was 6.4%, 3 times what they report for SVSs5). There are few studies which showed factors affecting the treatment result of CVSs for GKRS. The aim of this study was to confirm which factors affect the tumor progression after GKRS of CVSs.
MATERIALS AND METHODS
A consecutive series of 620 patients with VSs underwent GKRS between May 1990 and December 2013. Patients follow up less than 12 months by magnetic resonance image (MRI) were excluded. We used the GKRS to treat 511 patents affected by VSs, with median follow-up period 52 (range, 12–110) months. Group 1 defined as cyst/tumor volume >30%, group 2 was cyst/tumor volume >50%, and group 3 was solid tumor or cyst/tumor volume ≤30%.
All radiosurgery procedures in this study were carried out using a Leksell Gamma knife (B. and C. Elekta, Stockholm, Sweden). After a Leksell stereotactic coordinate frame was applied to the patient’s head, MR T1-weighted images with gadolinium contrast were obtained, and slices were reconstructed every 2 mm in the axial plane. GammaPlanElecta (Elekta) Instruments software was used to plan GKRS. The median tumor marginal dose was 12 Gy (range, 11–13.5 Gy) with a median isodose lines of 50% in all cases.
MRI was performed to evaluate the radiological response to GKRS every 6 to 12 months. We considered treatment outcome as regression, stable and progression, referred by generally accepted criterion9).All patients underwent preoperative magnetic resonance
RESULTS
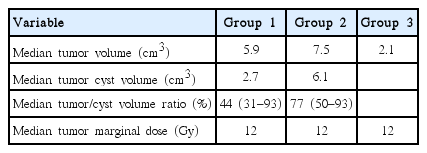
Group 1 included 8 male (29.6%) and 19 female (70.4%), with a median age of 59 at time of treatment (range, 38–79 years). Two patients had undergone previous incomplete resection, and one patient had prior ventriculoperitoneal shunt (VPS) placement. Group 2, selected from group 1, includes 3 male (33.3%) and 6 female (66.7%), with a median age of 60 at time of treatment (range, 44–79 years). One patient had undergone incomplete resection and one patient had prior VPS placement. Group 3 includes 164 male (33.9%) and 320 female (66.1%), with median age of 53 at time of treatment (range, 24–78 years). Fifty-one patients had undergone incomplete resection (Table 1).
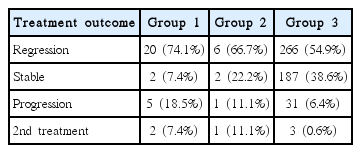
The efficacy of radiosurgery was assessed by observation of tumor’s maximal diameter changes in all 511 VS patients who underwent regular MRI follow-ups (Tables 2, 3). During the follow-up, 20 patients (74.1%) had a regression, 2 patients (7.4%) had a stable response, and 5 patients (18.5%) had a progression in group 1. In group 2, 6 patients (66.7%) had a regression, 2 patients (22.2%) had a stable response, and 1 patient (11.1%) had a progression. And in group 3, 266 patients (54.9%) had a regression, 187 patients (38.6%) had a stable response, and 31 (6.4%) had a progression. In group 3, when follow-up period was more than two years, progression was 24 (5%). Among increased tumor, two patients underwent surgical removal of the tumor in group 1, one and three in group 2, 3 respectively, due to combined neurological deterioration. The median interval time from GKRS to tumor resection was 51 months (32–72 months).
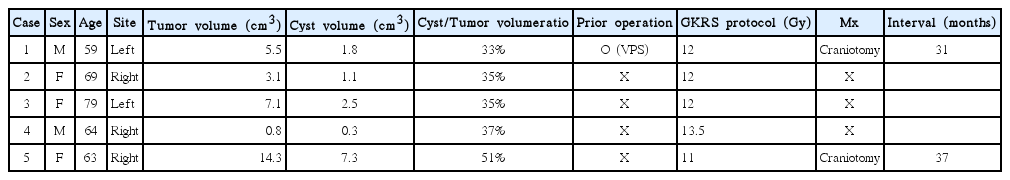
Table 4 shows progression tumors after GKRS in group 1. These five patients were female and right side dominant with median age 66.8 (range, 59–79 years) and one patient was also belong to group 2. Two underwent delayed microsurgery at 31 and 37 months after GKRS and the size of the tumor were 5.5 and 14.3 cm3, respectively. The histology study of the tumors showed typical benign VS in these two patients. Consider the median tumor volume in group 1, 2 and 3, we could conclude the reason for higher progression rate in group 1, 2 is higher tumor and cyst volume. Examples of MR scans for each groups are illustrated in Figs. 1, 2 and 3, respectively.
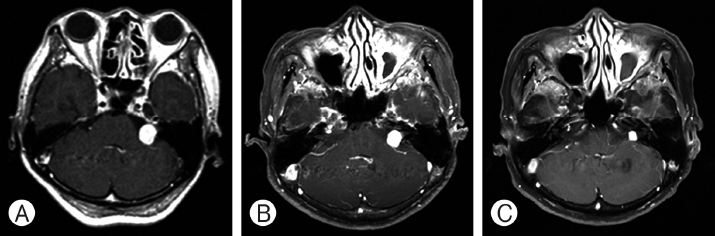
A series of magnetic resonanceimages, axial T1-weighted images with contrast, showing cystic mass, obtained in a 58-year-old female with tinnitus and vertigo at left cerebellopontine angle vestibular schwannoma. (A) Before the Gamma knife radiosurgery (GKRS), tumor volume was 2.5 cm3. (B) After 43 months, the tumor volume was decreased. (C) The tumor was more decreased after 94 months from GKRS.
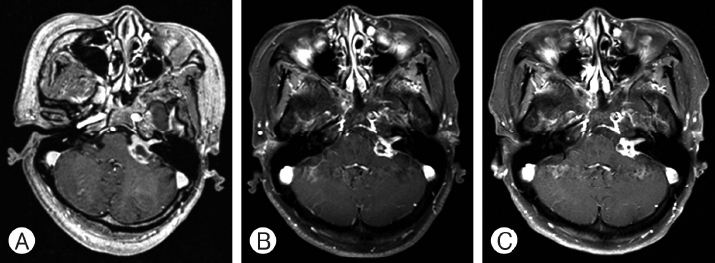
A series of magnetic resonanceimages, axial T1-weighted images with contrast, showing cystic mass, obtained in a 63-year-old female with hearing disturbance at right cerebellopontine angle vestibular schwannoma. (A) Before the Gamma knife radiosurgery (GKRS), tumor volume was 5.2 cm3 and cyst volume was 1.6 cm3. (B) After 14 months, the tumor and cyst volume was decreased. (C) The tumor was stable until 52 months from GKRS.
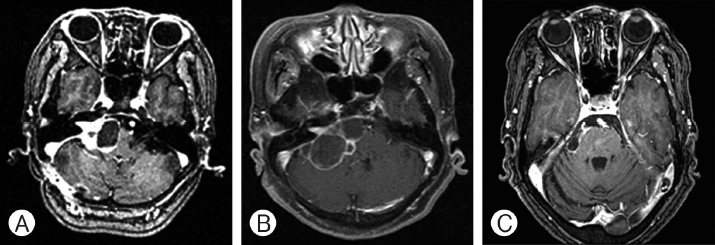
A series of magnetic resonance images, axial T1-weighted images with contrast, showing cystic mass enlargement after radiosurgery, obtained in a 63-year-old woman with right cerebellopontine angle vestibular schwannoma. (A) Before the Gamma knife radiosurgery, tumor volume was 14.3 cm3 and cyst volume was 7.3 cm3. (B) After 29 months, the cyst was further enlarged. (C) Craniotomy for tumor and cyst removal due to aggravated dizziness and facial nerve palsy.
DISCUSSION
CVS may represent a unique subtype of tumor. Generally CVSs are believed by some to have a more aggressive course with more rapid neurological deterioration and less predictable tumor than SVSs12). The pathological mechanism of cyst development in VS is unclear thus far. Isolated or repeated intratumoral hemorrhage, hyaline, fatty, and mucinous degeneration and microcystic change have all been proposed as possible mechanisms of cyst formation. Besides rapid progression, CVS was usually associated with an increased risk of operative damage to the facial nerve, surgery-related complications, and mortality compared with SVS1).
The biological mechanisms underlying the progression of CVS after GKRS are poorly understood. Possible mechanisms of CVS expansion after GKRS include osmotic mechanisms from vascular damage, which can induce extravasation of serum proteins into the extracellular matrix. Transient radiation-induced elevation in protein levels may also increase transudation from tumor vessels. Together, these factors increase the osmotic force favoring fluid accumulation in cystic spaces8). Charabiet al.3), mentioned that the formation and progression of cysts were due to the degeneration of tumor tissue. Ki-67 immunostaining of CVS revealed that rapid volume increase was more likely to due to an increase in the cyst volume than to a high growth rate of tumor cells3).
GKRS has become more popular during the last decade, and promising clinical results have been reported for treatment of small-to median sized VSs10). Published results of GKRS for the treatment of VS, treatment failure rate were 1.4%4) (defined as need for microscopic surgery) and 10%6) (defined as need for tumor enlargement). Compared with SVSs, CVSs tend to behave more unpredictably after GKRS, necessitating surgical removal. A limited number of studies on radiosurgery as the primary treatment for large or CVSs have been conducted, and microsurgery is generally considered the first choice2,5). Delsanti and Régis5) reported 54 CVSs treated with GKRS, and the failure rate (defined as need for a second procedure), was 6.4%, approximately 3 times what they reported for SVSs. In our study, the tumor progression rate of group 3 was 5% and group 1 was 18.5%. In group 3, 0.6% of patients needed salvage microscopic surgery because of tumor progression and 7.4% of patients in group 1, and 11.1% of group 2. This is similar to other studies’ results, and CVS shows higher treatment failure when treated with GKRS. For large or symptoms of mass effect and hydrocephalus are presents with VSs, microsurgery is the first option11). And it has been recently suggested that for asymptomatic patient, multisession stereotactic radiosurgery, compared with single-fraction radiosurgery, results in better hearing preservation and, in the case of larger VSs, fewer side effects due to better sparing of the normal tissues and organs at risk2).
CONCLUSION
In this study we found that GKRS for large CVS was associated with poor treatment outcome. Further study is needed to identify the mechanism of the tumor progression of CVS after GKRS.