Retrospective Analysis of Risk Factors for Recurrent Chronic Subdural Hematoma
Article information
Abstract
Objective
Recent meta-analyses propose burr hole (BH) drainage as the most efficient choice for treatment of chronic subdural hematoma (CSDH). But recurrence rate after BH drainage varies from 0.35% to 60%. In this study, we investigated the risk factors for the recurrence of CSDH after BH drainage.
Methods
We retrospectively reviewed 182 patients with CSDH treated by BH drainage. Univariate and multivariate analyses were performed to identify risk factors for recurrence of CSDH.
Results
Twenty patients (11%) underwent a reoperation because of the recurrence of CSDH during the study period. Liver disease (p=0.009), bilateral CSDH (p=0.034) and disturbance of consciousness (p=0.036) were related risk factors for recurrence of CSHD. Among them, liver disease (p=0.016) and bilateral hematoma (p=0.046) were independent risk factors for recurrence of CSDH by multivariate analysis.
Conclusion
Liver disease and bilateral hematoma were independent risk factors for recurrence of CSDH. If more data is collected, we can find independent factor for recurrent CSDH among liver disease.
INTRODUCTION
Chronic subdural hematoma (CSDH) represents an abnormal collection of liquefied blood degradation underneath the dura matter and usually forms in for approximately 3 weeks11). CSDH is one of the most common problems encountered in daily neurosurgical practice and the incidence is increasing8,27). The incidence rate of CSDH has been reported to be as high as 13.1 cases per 100,000 inhabitants. Karibe et al.6) reported an increase in the overall incidence of CSDH to 20.6/100,000/year, in the age group 70 to 79 years, and 127.1 in the age group over 80 years.
There are many treatment options for CSDH, recent meta-analyses propose burr hole (BH) drainage as the most efficient choice because it provides the best balance between recurrence and morbidity8,27). However, much controversy still remains regarding the recurrence rate, ranging from 0.35% to 60% at in the published data23). There are many reports about factors influencing recurrence, but controversial findings are not uncommonly reported2,14,18,28).
This objective of our retrospective study is to evaluate outcomes of BH drainage and risk factors for reoperation from our hospital8).
MATERIALS AND METHODS
This retrospective study analyzed a series of 182 consecutive patients with CSDH who were treated with BH trephination and drainage in our center between January 2012 and September 2014. We defined CSDH as a SDH surrounded by a thin capsule and consisted of dark reddish liquefied blood found at operation8). If the date of head trauma was clear, a CSDH is defined as a hematoma that had persisted more than 3 weeks after head trauma. The recurrence of CSDH is defined as a subsequent increase in hematoma volume in the subdural space for which reoperation was required of newly developed symptoms8,26). The data were collected and analyzed from the archives and protocols of the Asan Medical Center of Republic of Korea. Medical records as well as the pre and postoperative head computer tomography (CT) scans were reviewed retrospectively. In this study, hygroma, infantile CSDH, calcified or ossified CSDH, arachnoid cyst with CSDH were excluded because they were considered to be clinically different entities.
Baseline patients characteristics such as age, sex, history of head trauma, comorbidities, known risk factors for development of CSDH(use of anticoagulants or antiplatelet drugs, presence of coagulation disorder, a history of alcohol abuse and smoking) and clinical presentation at admission were analyzed8). We defined a history of alcohol abuse as the consumption of four or more alcoholic beverages per day. We analyzed other correlation factors related to recurrence, which were hematoma density (low, iso, high, mixed)8), hematoma location (unilateral, bilateral), width of hematoma, and pre-, postoperative midline shift. We defined bilateral CSDH when CSDH seen on both side of at the CT scan. And there were no patient who received bilateral BH trephination.
All patients underwent one BH trephination at the site of its maximal hematoma thickness under local or general anesthesia. A silicon tube was inserted through a small hole opened in the outer hematoma membrane and connected to a closed drainage system8). The drainage tube was maintained about head level and usually removed after 2 to 3 days, after checking the results of a follow up brain CT scan. All patients were follow up for 3 or more months until the disease was regarded as in remission8).
Statistical analysis was performed with Pearson’s chi-square test and student t-test to assess the relationship between each variable and the recurrence of CSDH. All significant univariate results were then tested in multivariate logistic regression model. The statistical significance was set at p<0.05.
RESULTS
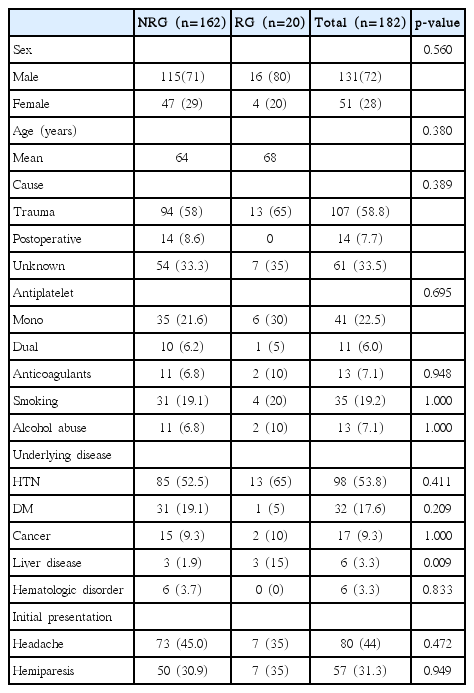
Table 1 shows the baseline characteristics of the patients with CSDH and the results of the univariate analysis of the relationship between the recurrence of CSDH and each risk factor. There were 131 males (72%) and 51 females (28%) in the study, ranging in age from 10 to 93 years (median age, 67 years). Twenty patients (11%) experienced recurrence. There were 16 males (80%) and 4 females (20%), ranging in age from 10 to 93 years (median age, 73 years). Mean age of patients in the recurrence group (RG) (68) was not significantly different from that in the non-RG(NRG) (64). Male predominance was found in our operative database. 107 patients (58.8%) had remembered their initial head trauma and ranged from 3 weeks to 4 months for the patients with the history of initial trauma to be made as CSDH. The demographic data and cause of CSDH were not significantly associated with the recurrence of CSDH. The only underlying disease related to the recurrence of CSDH was liver disease (p=0.015). No initial clinical manifestation was related to the recurrence of CSDH. We did not detect significant differences between CSDH recurrence and current antiplatelet or anticoagulant therapy.
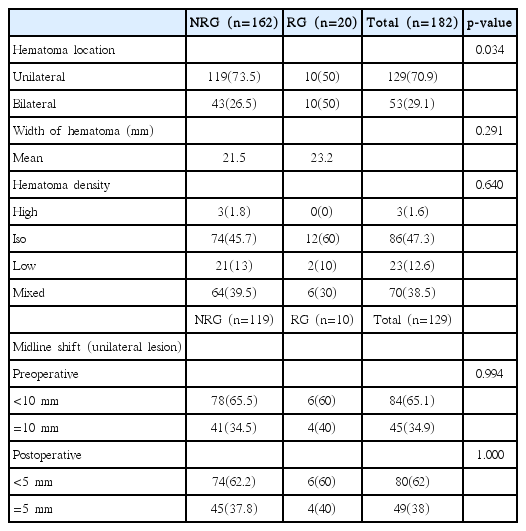
Table 2 shows the results of a comparison of radiological factors between the two groups. The recurrence of CSDH occurred on bilateral CSDH in 10 patients (50%) and unilateral CSDH in 10 patients (50%), which showed significant difference in relation to location between the two groups (p= 0.034). Preoperative and postoperative midline shifting, hematoma depth, hematoma density and degree of brain atrophy were not significantly associated with the recurrence of CSDH. Patient with CSDH recurrence tended to have a history of liver disease and bilateral CSDH.
Table 3 shows the results of multivariate logistic regression analysis. We found that liver disease (odds ratio [OR], 12.666; 95%; p=0.016) and bilateral hematoma (OR, 2.719; 95%; p= 0.046) were independent risk factors for the recurrence of CSDH.
DISCUSSION
CSDH is a common disease in neurosurgical practice and the incidence is increasing27). For treatment of CSDH, “minimally invasive” approaches, such as BH trephination is selected as initial treatment of choice by many hospitals because of its simplicity and the lower operative risks29). However it has been reported that recurrence rate range from 2.3% to 27%9,25) where this study showed a recurrence rate of 11%.
The etiology of recurrence of CSDH has not been completely understood until now10), but several risk factors for recurrence of CSDH have been reported, including advanced age, brain atrophy, bilateral CSDH, hematoma density, seizures, diabetes mellitus, bleeding tendency, alcohol abuse, and postoperative posture3,13,15–17,24,26,30). However, the definitive risk factors have not been defined until now. The purpose of in this study was to identify risk factors for reoperation of recurrent CSDH, which were liver disease, bilateral lesions and disturbance of consciousness at initial presentation.
1. Liver Disease
We defined liver disease as liver cirrhosis caused by any causes (hepatitis B virus, hepatitis C virus, cardiac cirrhosis, alcohol and so on) and liver cirrhosis as diagnosed by liver ultrasound or liver CT scan; six patients who were included in liver disease criteria. Three of them experienced recurrent CSDH, relapse two out of three patients with no history of trauma. According to child’s classification, 3 of them were A, 2 patients were B, and 1 patient was C. Usually liver cirrhosis is a well-known risk factor for spontaneous intracranial hemorrhage4). Chen et al.1) reported that the surgical complication rate was 43.2%, the rebleeding rate was 36.4%, and the mortality rate was 22.7%. Cirrhosis-related complications may be associated with acquired thrombocytopenia and coagulopathy resulting from hypersplenism, impaired liver function with decreased fibrinogen and increased fibrinolysis, damaged systemic vessel walls and deficient platelet aggregation, the induction of hypertension, and activation of the clotting cascade4,7,12). Therefore, we could think that liver disease can be a risk factor for recurrent CSDH. If more data is collected, we can find independent factor for recurrent CSDH among liver disease.
2. Bilateral CSDH
The overall incidence of bilateral CSDH has been reported to vary from 16% to 20%20,22), the present data shows 28% of bilateral CSDH. Several papers reported that presence of bilateral CSDH was identified as a risk factor for a recurrence of CSDH21,22,26). Poor postoperative re-expansion of the brain is considered the main reason for hematoma recurrence13,15). This results in the persistence of an enlarged subdural space in patients undergoing evacuation of a CSDH creates potential for reaccumulation of the hematoma, recurrent CSDH3,10). Patients with bilateral CSDH tend to have previous brain atrophy, with may lead to poor brain re-expansion after the operation. Oyama et al.19) reported that bilateral CSDH occurred more frequently in patients with prolonged coagulation time. Whether coagulopathy or other types of bleeding tendency predisposes to the higher recurrent rate found in the patients with bilateral CSDH is unknown. Further studies are required to evaluate the association between recurrence and bilateral CSDH.
3. Disturbance of Consciousness
Clinical symptoms of CSDH appear to vary depending on the degree of intracranial pressure from a symptomatic to comatous. Besides, headache, speech, sensorimotor disturbance, altered behavior, or seizure may occur6). In this paper, the most common initial clinical presentation were headache and hemiparesis. Although it did not get the statistical significance in multivariate logistic analysis, disturbance of consciousness tended to be associated with recurrence of CSDH. There is a report, that disturbance of consciousness was a predictor of unfavorable outcomes after treatment of CSDH5). So, more data collection and study is needed to identify the association between the disturbance of consciousness and the recurrence of CSDH.
Because of this study was a retrospective study, it is potentially subject to sources of bias and variation. We conducted an analysis of the effect of alcohol abuse, but there is a possibility that the information provided by the patient was not correct. We believe that a large prospective study with a good follow-up rate is desirable.
CONCLUSION
CSDH is common neurosurgical problems and a recurrent rate after surgical treatment is common. Liver disease and bilateral CSDH were independent predictors for the recurrence of CSDH after BH trephination. This information might be helpful for predicting the recurrence of CSDH after BH trephination.