Cervical Myelopathy Caused by a Ventriculoperitoneal Shunt: A Case Report
Article information
Abstract
Intracranial hypotension is a rare cause of cervical myelopathy. Cerebrospinal fluid (CSF) shunt surgery, which belongs to the broader category of ventriculoperitoneal shunt surgery, is commonly chosen as the primary treatment option for hydrocephalus. However, such surgery can potentially lead to CSF overdrainage, resulting in the occurrence of intracranial hypotension. In the present case, spinal surgery was performed twice for cervical myelopathy; however, there was no improvement in symptoms such as gait disturbance. After the readjustment of the shunt valve pressure, the symptoms improved.
INTRODUCTION
Intracranial hypotension, also referred to as cerebrospinal fluid (CSF) hypovolemia, is the result of reduced CSF volume or pressure. The cause is typically CSF leakage, which can occur when the dura mater is damaged by trauma, lumbar puncture, craniotomy, spinal surgery, or CSF shunt placement8,11).
CSF shunt surgery is one widely used option for treating hydrocephalus. However, inappropriate shunting CSF can induce CSF overdrainage and intracranial hypotension13). This can cause some complications, such as subdural hematoma, slit ventricle syndrome, and postural headache. Common symptoms in those with intracranial hypotension are postural headache and headache accompanied by nausea, vomiting, and dizziness5,10). Less common symptoms include hearing problems, seizures, facial numbness, and so on8). The syndrome can potentially cause unusual or infrequent symptoms, which can be attributed to alteration or compression of structures within the brain or spinal cord3). Myelopathy by upper cervical cord compression is one of the rare manifestations in cases of intracranial hypotension by engorgement venous complex. Recent studies have referred to these symptoms as “overshunting-associated myelopathy (OSAM)”1,5,6).
With this background, we report unusual and confusing cervical myelopathy caused by intracranial hypotension with engorgement of epidural venous plexus and cervical cord compression.
CASE REPORT
A 55-year-old man presented with nausea, progressive gait disturbance, hand clumsiness, and whole extremities weakness beginning one month ago. He received an aneurysm neck clipping and ventriculoperitoneal shunt 15 years prior due to spontaneous subarachnoid hemorrhage and hydrocephalus, after which he could walk without assistance. His modified Rankin scale score was 1. The patient spent years without problems after the operation.
In the examination of motor function, he demonstrated 4/5 strength in his both upper and lower extremities. He also showed sensory deterioration of about 7/10. The shunt was a programmable valve shunt with a shunt pressure of 190 mmH2O. Despite the valve pressure, the ventricle size appeared slit-like, initially suggesting overdrainage due to valve malfunction. Simultaneously, an engorged vein and epidural hematoma were observed on cervical spine magnetic resonance imaging (MRI) on cervicomedullary junction with reduced size of spinal canal. There was also strong enhancement in the lesion with direct compression of spinal cord from both lateral aspects at the C1-2 level mixed with prominent venous plexus (Fig. 1). We suspected spinal arteriovenous fistula (AVF) as the cause of the epidural hematoma at the C1-2 level, but no dural AVF was detected on brain MRI or digital subtraction angiography (DSA) performed at another hospital. Therefore, myelopathy was suspected to be caused by the prominent venous plexus and epidural hematoma, considering the patient's medical history, OSAM was initially suspected. We thought shunt valve replacement is necessary to prevent overdrainage caused by shunt malfunction. Additionally, the patient was currently experiencing gait disturbance, so we thought removal of the hematoma at the C1-2 level and decompression are needed. C1-2 level decompressive laminectomy and replacement of a new programmable valve were performed and hematoma evacuation was carried out. After removing the C1 posterior arch, an engorged vein and dark-colored hematoma were observed. The pressure of the replaced valve was initially set at 150 mmH2O. His symptoms were expected to be improved.
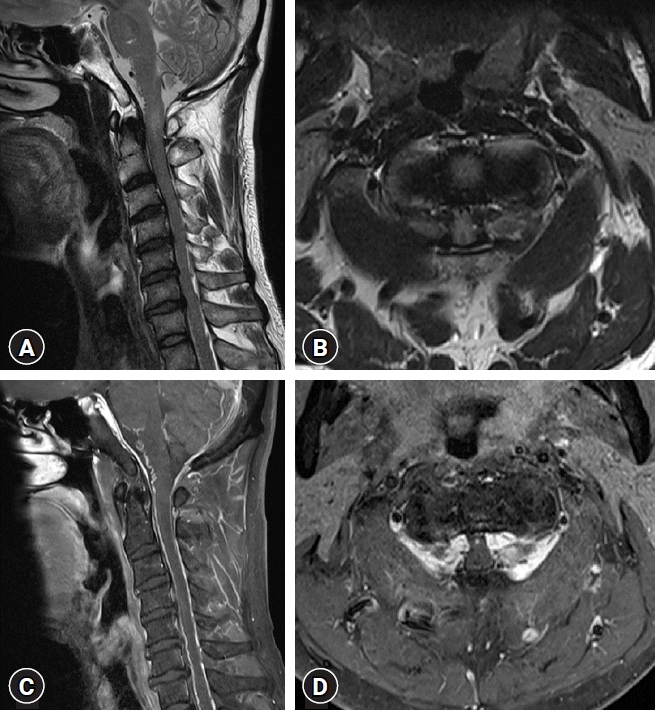
Cervical spine magnetic resonance imaging with enhancement. (A) T2 sagittal image, (B) T2 C1-2 level axial image, (C) contrast-enhanced T1 sagittal image, (D) contrast-enhanced T1 axial C1-2 level image. (A, B) Low signal intensity at the cervicomedullary junction and low signal intensity space-occupying lesions from both lateral aspects of the spinal canal. (C, D) Lesions with direct compression of the spinal cord from both lateral aspects, mixed with a prominent venous plexus and an organized epidural hematoma.
However, the patient still exhibited lower limb weakness and gait disturbance. Since decompression of the cord had been achieved (Fig. 2), a postoperative brain computed tomography scan showed an increase in ventricle size (Fig. 3). Considering the persistent gait disturbance caused by worsening of hydrocephalus, the valve pressure was gradually lowered to 80 mmH2O. Despite lowering it to 80 mmH2O, the patient's gait disturbance persisted and the ventricle size remained unchanged. To explore other potential problems, electromyography was performed. However, no definite abnormalities were observed.

Postoperative cervical spine magnetic resonance imaging, revealing that the previous lesion causing cord compression has disappeared and decompression has been achieved. (A) T2 sagittal image. (B) T2 axial image at the C1-2 level.

(A) Brain computed tomography (CT) scan taken before surgery, showing a slit-like appearance of the ventricle size. (B) CT scan taken after surgery, following the insertion of a valve adjusted to a shunt pressure of 150 mmH2O, revealing an increase in ventricle size.
The patient's symptoms were consistent with myelopathy and lower cervical spinal stenosis observed on the cervical MRI. Surgical treatment for spinal cord compression in the lower segment was continued. A decompressive laminectomy and posterior cervical fusion from C3 to C6 was performed. Although walking was difficult without assistance, the patient subjectively reported improved lower limb strength. Rehabilitation therapy was initiated, and the patient's progress was monitored.
During follow-up, an engorged vein at the C1-2 level was again visible, suggesting cord compression (Fig. 4). The patient complained of worsening symptoms after discharge. DSA was performed, showing no abnormalities such as AVF. Returning to the initial assumption, symptoms were thought to be caused by intracranial hypotension. The patient remained hospitalized and the valve pressure was gradually increased to 100 mmH2O and 120 mmH2O. One week after increasing the pressure to 120 mmH2O, the patient reported improvement in lower limb weakness and an ability to walk without assistance. An outpatient cervical spine MRI obtained after discharge showed improved central stenosis at C1-2 due to resolution of the engorged vein (Fig. 5).

Cervical spine magnetic resonance imaging taken during follow-up, revealing the reappearance of a previously absent space-occupying lesion at the C1-2 level, which was observed to cause cord compression. (A) T2 sagittal image. (B) T2 axial image at the C1-2 level.

Cervical spine magnetic resonance imaging taken during outpatient follow-up, showing disappearance of the previously observed space-occupying lesion at the C1-2 level. These images were obtained after progressive incrementation of the shunt valve pressure and subsequent monitoring. (A) T2 sagittal image. (B) T2 axial image at the C1-2 level.
DISCUSSION
Headache is the predominant symptom among patients experiencing intracranial hypotension, although they may also exhibit various other symptoms such as neck pain, vomiting, dizziness, changes in hearing or vision, visual field cuts, cranial nerve palsies affecting the third, fourth, and sixth nerves, weakness in the bulbar region, labyrinthine hydrops, galactorrhea, pain between shoulder blades, radiculopathies, parkinsonism, ataxia, frontotemporal dementia, encephalopathy, and even coma4).
Cervical myelopathy caused by engorged epidural veins resulting from CSF overdrainage is a rare complication of shunt procedures. It is influenced by the intricate interplay of fluid dynamics and the unique craniospinal anatomy13). According to the Monro-Kellie doctrine, any reduction in CSF volume must be compensated for due to the skull's non-compressible nature. Consequently, decreased CSF volume can lead to increased intracranial blood volume, primarily affecting the venous system. This can result in diffuse meningeal venous hyperemia and engorgement of the venous sinuses, which can be observed as dural enhancement and thickening on MRI. Unlike the adherent dura within the skull, the spinal canal contains an epidural space housing the epidural venous plexus. This plexus consists of a valveless network of veins that connect superiorly with cranial venous drainage through the marginal sinus and inferiorly with veins along the spinal cord1).
Spinal manifestations resulting from intracranial hypotension have been observed in approximately 6% of patients. They can affect various levels of the spine, leading to either myelopathy or radiculopathy4). The diagnosis of this condition is challenging and often delayed due to infrequent evaluation of spinal imaging, compounded by the fact that nearly 20% of brain MRIs appear normal4). Brain MRI findings associated with intracranial hypotension can vary. They may include diffuse pachymeningeal enhancement, subdural fluid collection, and engorged cerebral venous sinuses. On the other hand, spinal MRI may reveal extra-arachnoid fluid collection, extradural fluid extravasation, meningeal diverticula, diffuse pachymeningeal enhancement, and engorgement of the spinal epidural venous plexus. Spinal cord compression resulting from intracranial hypotension was first reported by Miyazaki et al.9). In their case, axial MRI revealed compression of the spinal cord from both lateral aspects due to engorged venous plexus. Similar findings of engorged vein structures were observed in our case, suggesting the possibility of CSF overdrainage in the presence of spinal cord compression.
Compensatory engorgement of epidural venous plexus can easily be managed by controlling shunt pressure. To date, there have been a number of reports of cervical myelopathy caused by intracranial hypotension. Recent literature has termed these symptoms OSAM1). The majority of patients presented with motor deficit clinical symptoms (11 cases). Only one case presented with neck and back pain. In most reported cases, symptoms were improved after a shunting operation, like shunt revision or changing from a one-way valve mechanism system to a programmable valve system1,5-9,12,13). In some cases, the shunt is removed and symptoms are improved2).
CONCLUSION
CSF overdrainage can lead to cervical epidural vein engorgement, which, in turn, may cause cord compression. Neurosurgeons should be aware of the possibility of cervical myelopathy in such situations and diagnose it accordingly. If the patient exhibits myelopathy symptoms and has a history of shunt surgery, consideration should be given to the potential occurrence of myelopathy resulting from these factors.
Notes
No potential conflict of interest relevant to this article was reported.