INTRODUCTION
Glioblastoma, which is predominantly an intra-axial neoplasm, is the most common primary malignant brain tumor4). Glioblastoma may exhibit characteristic radiological features of other diseases, such as arteriovenous malformations, viral encephalitis, lymphomas, subacute strokes, and cerebral abscesses, upon isointensity (computed tomography [CT]) or magnetic resonance imaging (MRI). However, glioblastomas mimicking the radiological features of a meningioma are rare. Herein, we describe a patient with glioblastoma exhibiting the radiological features of a meningioma.
CASE REPORT
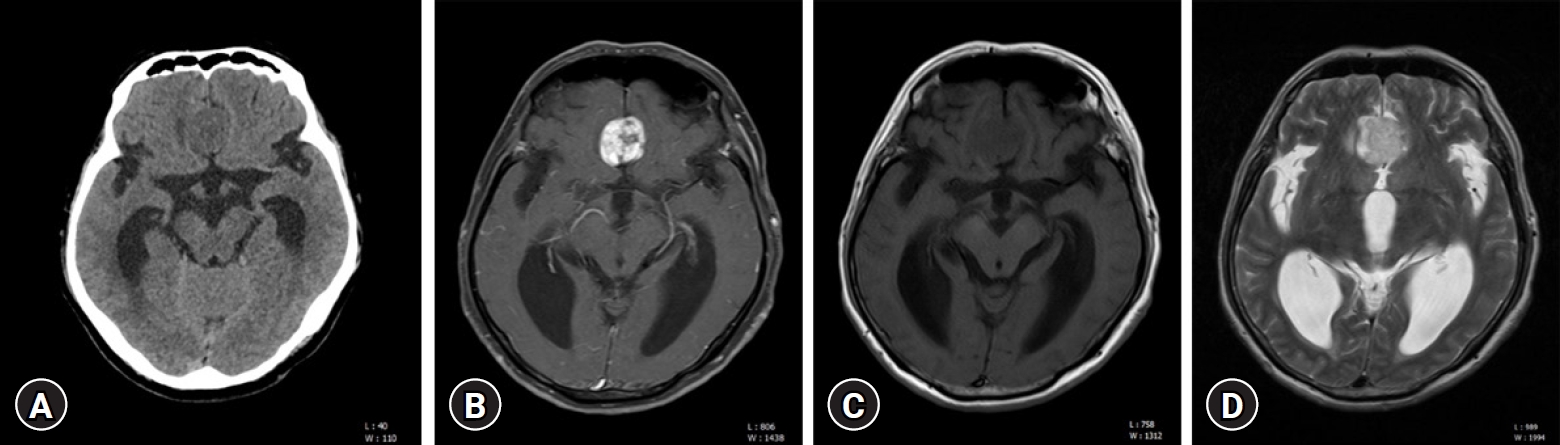
A 55-year-old male patient presented with gait disturbance, mild dysarthria, and dizziness over two months. CT imaging revealed ventriculomegaly and a discernible mass in the interhemispheric fissure (Fig. 1A). Subsequent MRI scans identified a heterogeneously enhancing extra-axial mass, measuring 2.3×2.3×2.5 cm, with well-defined margins (Fig. 1B). Isointensity to the gray matter in the T1-weighted sequence (Fig. 1C) and slight hyperintensity relative to the gray matter in the T2-weighted sequence (Fig. 1D) were observed. Notably, the T2-weighted sequence demonstrated a cerebrospinal fluid (CSF) cleft (Fig. 1D) between the tumor and adjacent brain cortex, leading to an initial radiological diagnosis of meningioma. Two days later, the patient underwent a bifrontal craniotomy for tumor resection, which revealed a highly vascularized, yellowish-gray, friable mass within the interhemispheric fissure (Fig. 2). Macroscopic examination revealed no significant infiltration of the tumor into the brain parenchyma, and subtotal mass removal was performed. Intraoperative pathological assessment suggested a high-grade glioma. Histopathological examination of the surgical specimen revealed atypical glial cells and marked endothelial and microvascular proliferation, but no necrotic changes (Fig. 3A, B). Immunohistochemical analysis demonstrated diffuse positivity for S-100, phosphatase and tensin homolog, and glial fibrillary acidic protein in the tumor cells (Fig. 3C). In contrast, the tumor cells were negative for epithelial membrane antigen, aiding in the differentiation of the specimen from a meningioma. The proliferation index, as indicated by Ki-67, was intermediate, ranging between 16% and 30%. Molecular investigations revealed no O-6-methylguanine-DNA methyltransferase gene methylation or isocitrate dehydrogenase (IDH) 1 mutations (Fig. 3D). These findings were consistent with those of glioblastoma, IDH wild-type, and World Health Organization (WHO) grade IV. Postoperative MRI, conducted one day after surgery revealed no residual enhanced lesions (Fig. 4). Subsequently, the patient underwent whole-brain radiotherapy and chemotherapy with temozolomide, and was discharged without any neurological deficits. The patient remained symptom-free for approximately five months after surgery.
DISCUSSION
Definitive diagnosis of an intracranial mass depends on pathological and molecular analyses. However, radiological characteristics serve as the primary means of gauging the nature of the tumor prior to performing a biopsy. Determining whether intracranial masses are intra- or extra-axial is very important as this affects the diagnosis, treatment plan, and prognosis. Extra-axial masses include meningiomas, schwannomas, metastatic lesions, arachnoid cysts, epidermoids, dermoids, chordomas, and eosinophilic granulomas5,15). The characteristic radiological features differentiating extra-axial from intra-axial masses include local bone changes, white matter buckling (inward compression of the gray-white junction), pseudocapsule (signal void of the dura or displaced vessels), CSF cleft (CSF or cortical vessels entrapped between the tumor and underlying cortex), and dural tail sign (enhancement of the dura infiltrating away from the lesion)2,5,6,9,15).
Glioblastomas typically present as invasive intra-axial masses with irregular margins. The typical characteristics of glioblastomas are hypodense signal change on the T1-weighted sequence and hyperintense signal change on the T2-weighted sequence. On contrast-enhanced T1-weighted MRI scans, glioblastomas classically appear hypointense to isointense, displaying a ring-enhancing pattern, often accompanied by vasogenic edema, central necrosis, and infiltration into the white matter nerve bundles1). Conversely, meningiomas generally appear as lobular, extra-axial masses with well-defined margins, exhibiting avid, homogeneous enhancement on contrast-enhanced T1-weighted MRI scans, although they may occasionally present with areas of central necrosis or calcification that do not enhance14,18). The typical characteristics of meningiomas are iso to slight hypodense signal change on the T1-weighted sequence and iso to hyperintense signal change on the T2-weighted sequence. Distinct radiological features, such as the dural tail sign, CSF cleft sign, sunburst or spoke-wheel appearance of vessels, and either osteolysis or hyperostosis, are indicative of meningiomas13,18).
In the present case, the patient's MRI findings— a lobular, extra-axial mass with a well-circumscribed margin, and CSF cleft sign—were consistent with a meningioma than a glioblastoma. Therefore, even if the radiological findings are consistent with those of a meningioma, the possibility of a glioblastoma should not be excluded.
The WHO 2016 Classification of Tumors of the Central Nervous System (WHO CNS4) categorizes astrocytic tumors into astrocytomas, either IDH mutant or wild type, and glioblastomas, also IDH mutant or wild type. However, the WHO 2021 Classification of Tumors of the Central Nervous System (WHO CNS5) reclassified astrocytic gliomas into astrocytoma, IDH-mutant, and glioblastoma, IDH-wild type. IDH-mutant astrocytomas are categorized as CNS WHO grades 2, 3, or 4 in the WHO CNS5, whereas IDH-wild type astrocytomas, synonymous with the diagnosis of glioblastoma, are classified as grade 4. The WHO CNS4 classification of glioblastoma, IDH-mutant (secondary glioblastoma), and IDH-wild type (primary glioblastoma) was updated to separate entities: astrocytoma, IDH-mutant, and glioblastoma, according to the WHO CNS5. Table 1 summarizes the differences between the WHO CNS4 and CNS510).
To our knowledge, few cases of glioblastoma-mimicking meningioma have been reported. As mentioned above, the diagnostic criteria for glioblastomas have changed. However, reports published in the literature can still be used as a reference. Dabboucy et al.3) reported a case of primary extra-axial glioblastoma in the left temporal area that exhibited a dural tail and CSF cleft sign. Patel et al.14) described two cases of glioblastoma mimicking meningioma. The first case involved a heterogeneously enhancing right temporoparietal mass with extensive contact along the right tentorium, a CSF cleft sign, and a dural tail sign, while the second case involved a left parasagittal, heterogeneously enhancing mass adjacent to the falx, and featured a dural tail sign. Taghipour Zahir et al.16) reported a glioblastoma with calvarial involvement. Lee et al.12) described a case of a glioblastoma within the cerebellopontine angle, while Karthigeyan et al.7) reported a case of glioblastoma within a petroclival location. Kayaci et al.8) and Varma et al.17) described cases of glioblastomas in the mid one-third falx. Table 2 summarizes the reported cases of glioblastomas mimicking meningiomas.
Magnetic resonance spectroscopy (MRS) can be used to distinguish between benign and malignant tumors, thus assisting in diagnosis. In a study by Lee et al.12), MRS was utilized for cerebellopontine angle tumors that rapidly increased in size over three months. The findings demonstrated increased choline-to-creatinine ratios and lactate levels, whereas N-acetyl aspartate levels were decreased, suggesting malignancy. Similarly, Karthigeyan et al.7) conducted MRS on a petroclival tumor in a 27-year-old patient with a relatively short disease duration and rapidly progressing symptoms. Although the patient's CT scan showed calcification, MRS revealed a choline-to-creatinine ratio of 2.5, indicating malignancy11). In cases exhibiting symptoms suggestive of malignancy, as in the two cases mentioned above, differentiating the tumors using MRS appears beneficial, even if the radiological images suggest a benign nature. However, when symptoms and imaging findings lean towards benign indications, the use of MRS may require further contemplation, although it undoubtedly contributes to making the diagnosis.
CONCLUSION
Glioblastomas may present with radiological features similar to those of meningiomas, making it precise diagnoses and formulating treatment plans challenging for physicians. Additional diagnostic tests, such as MRS, can be instrumental in aiding the diagnosis; however, their clinical indications require further deliberation.